Method for producing human collagen type II
A technology of human collagen and fluorescent protein, applied in the field of production of recombinant human collagen type II, which can solve the problems of inability to carry out large-scale production, low expression level, and hidden dangers of viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: the acquisition of recombinant plasmid
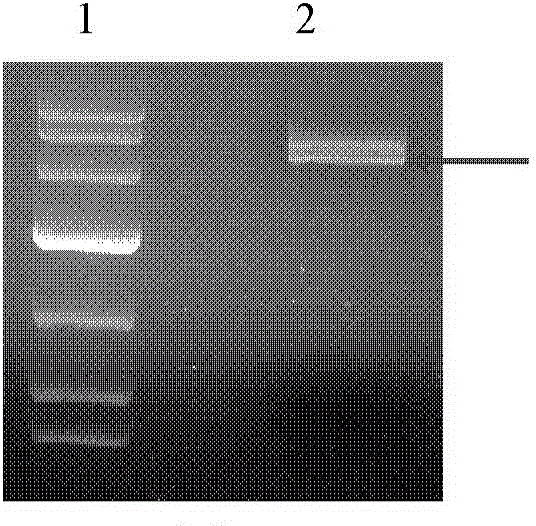
[0080] According to the polymorphism of EX-5512-B31 (synthesized by GeneCopoeia, Accession No.: NM_033150, its ORF sequence is shown in SEQ ID No.4, which contains the full-length sequence of human collagen type II (SEQ ID No.1)) Cloning site, select restriction site Xba I and Avr II to carry out double enzyme digestion on EX-5512-B31 to obtain the desired target gene, that is, human collagen type II α1 chain full-length gene (sequence such as SEQ ID No .1). And the genes IRES (sequence shown in SEQ ID No.5) and mCherry (sequence shown in SEQ ID No.6) were synthesized. Then, the human collagen type II α1 chain full-length gene (sequence shown in SEQ ID No.1) was connected to the transfer vector pFBDM (purchased from Shenzhen Baozhu Biotechnology Co., Ltd., Cat. No. zt323), construct pFBDM-col II, link the synthetic IRES and mCherry into pFBDM-col II, and construct the recombinant plasmid pFBDM-IM-col II.
[0081]...
Embodiment 2
[0082] Example 2: Recombinant Human Collagen Type II Virus Infects BmN to Construct Expression Vector Virus
[0083] The recombinant plasmids pFBDM-IM-col II and pUCDM-P4Hα-P4Hβ constructed in Example 1 were introduced into the asd auxotrophic host Escherichia coli SW106 by transposition (this laboratory preserved and transformed, and the transformation methods refer to Sun, Jingchen et al, Production of recombinant Bombyx mori nucleopolyhedrovirus in silkworm by intrahaemocoelic injection with invasive diaminopimelate auxotrophic Escherichia coli containing BmNPV-Bacmid, Biotechnol. Appl. Biochem. (2010) 57, 117-125 (Printed in Great Britain. 20BA) doi 04: The recombinant genetically engineered bacteria SW106-pFBDM-col II-IM-pUCDM-P4Hα-P4Hβ producing human collagen type II.
[0084] In LB-Kan-Tet-Spe-Gm-Cm-DAP solid medium (medium composition: ordinary LB medium, Kan-Tet-Spe-Gm-Cm-DAP is antibiotics and nutritional ingredients, which contains 50ug / ml Kanamycin (Kan), 10ug / ml...
Embodiment 3
[0086] Example 3: Expression of Collagen Type II by Virus Injection Bombyx mori
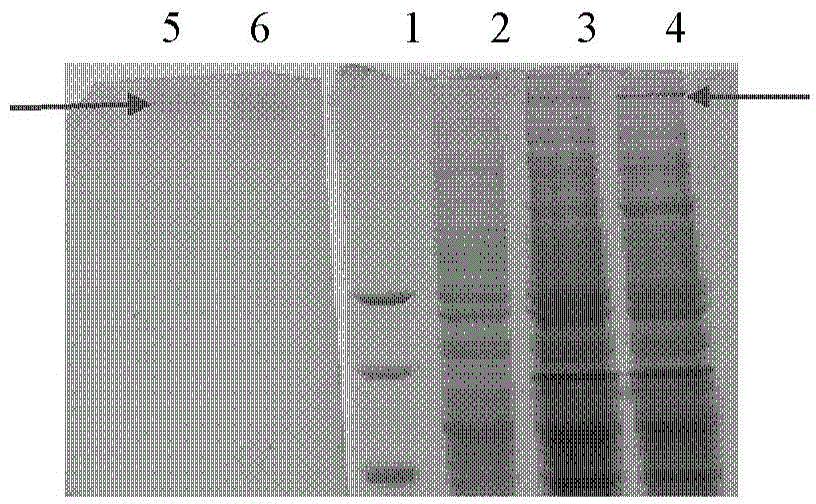
[0087]The recombinant Escherichia coli successfully transformed in Example 2 is directly infected with silkworm cell BmN (ATCC CRL-8910) in Grace medium (purchased from Gibco), and can produce baculovirus BmMNPV-hcol II, and the virus supernatant is 12000rpm Centrifuge for 1 minute to remove residual BmN cells and take the supernatant. Inject the virus supernatant into the penultimate segment of the 5th instar larvae of the silkworm with a micro-injection syringe, inject 10ul per silkworm, 28°C, humidity 60% to 70%, after feeding for 4 days, select the epidermis under the observation of a stereofluorescence microscope to show red fluorescence (caused by mCherry) of the silkworm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com