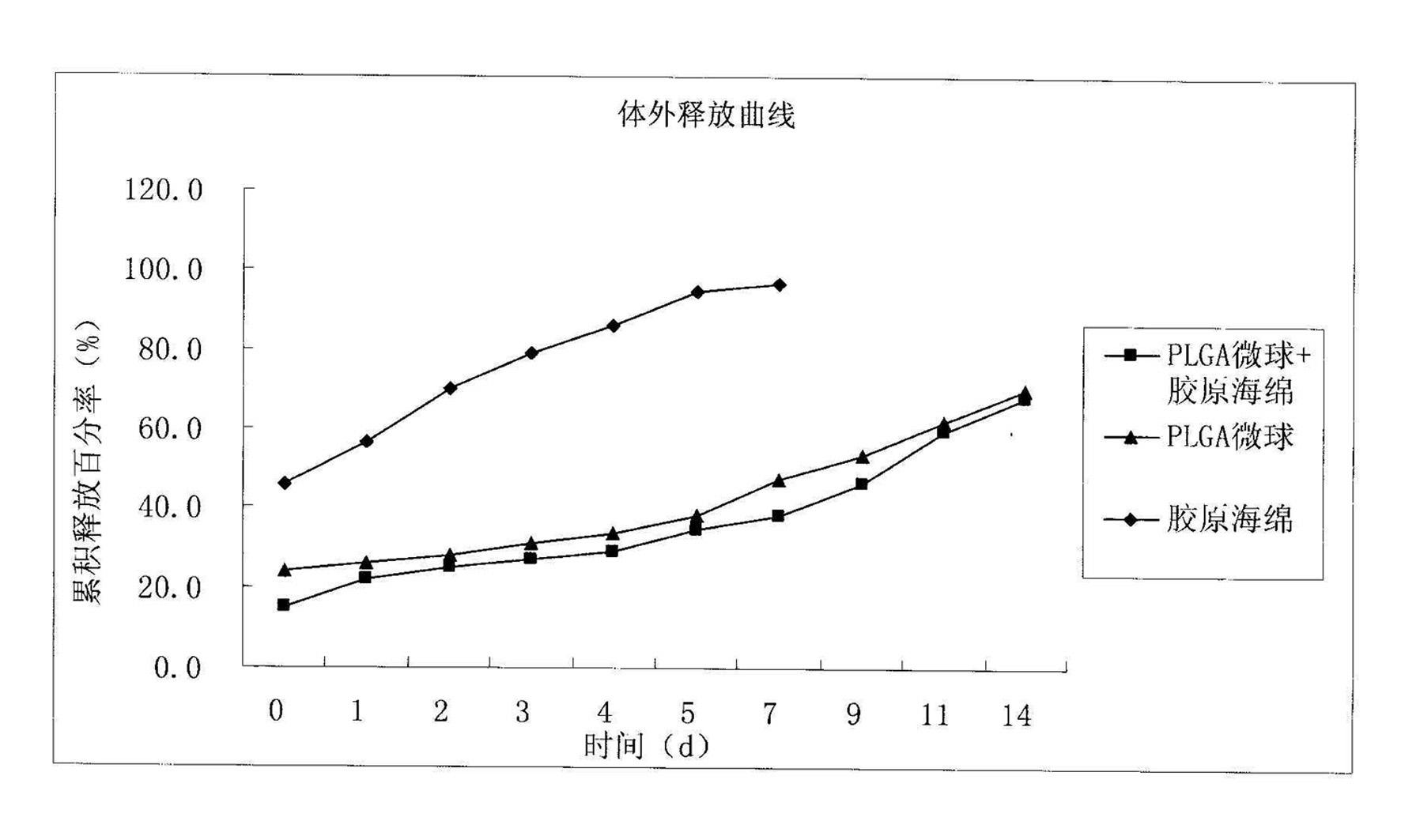
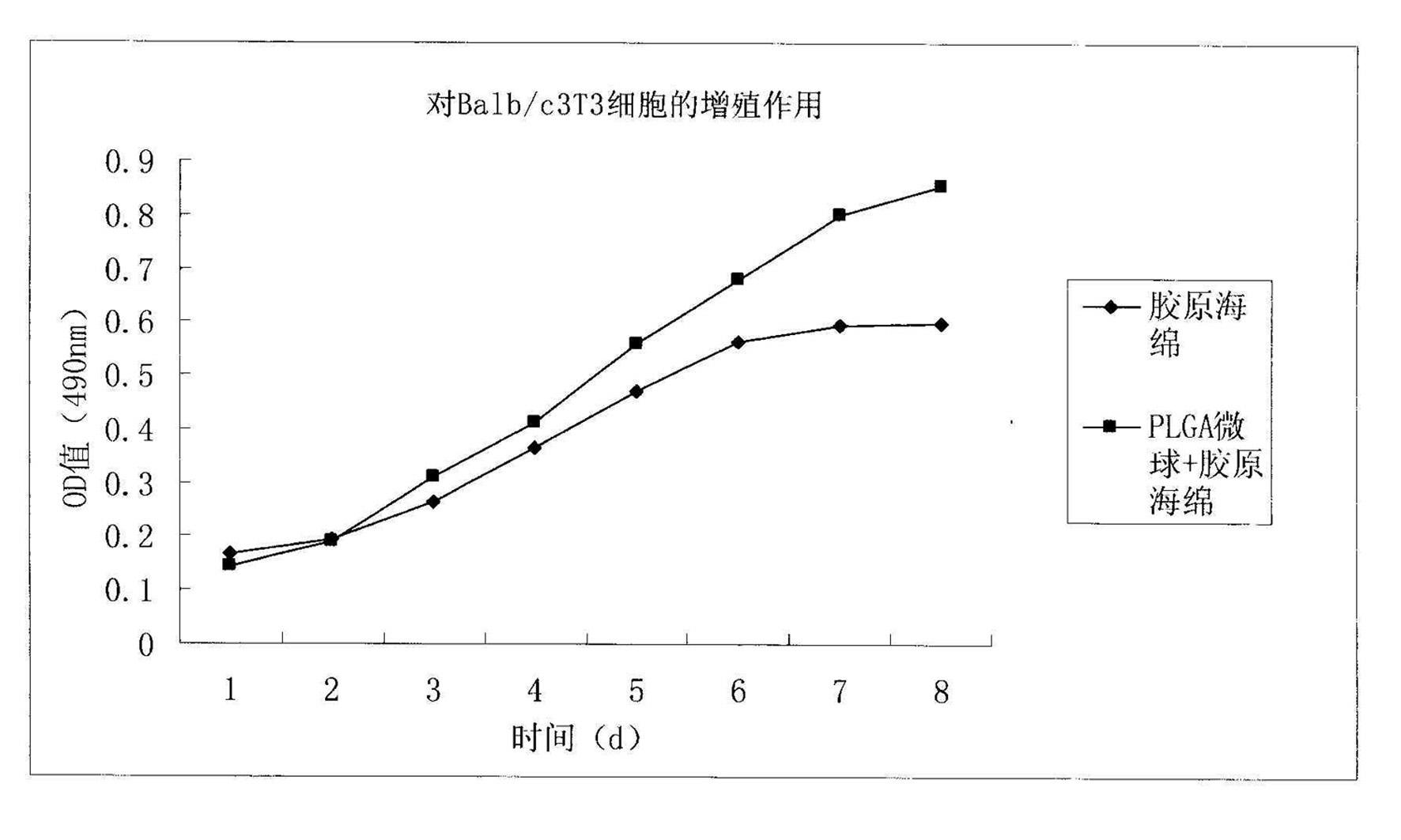
Method for preparing basic fibroblast growth factor sustained-release carrier
A technology of fibroblasts and slow-release carrier, which can be applied to medical preparations containing active ingredients, pharmaceutical formulas, peptide/protein components, etc. wait for the question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1. Dissolve 100mg of PLGA (PLA:PLG=50:50) in dichloromethane as the oil phase, dissolve 70ug of bFGF sample in double distilled water as the water phase, then add the bFGF solution into the oil phase and homogenize Then colostrum is formed. Add 40ml of 1% PVA solution, through the action of high-speed milk homogenizer (20000rpm, 2min) to form W / O / W, remove the organic solvent by rotating under reduced pressure with a rotary evaporator, centrifuge to precipitate the microspheres and freeze-dry to obtain the encapsulation PLGA Microspheres of bFGF.
[0020] 2. Add type I collagen to 0.15% acetic acid solution, stir well, and prepare collagen solution.
[0021] 3. Add the microspheres prepared in step 1 to the collagen solution in step 2, stir evenly, add to the mold (or a 96-well plate), pre-freeze at -70°C for 24 hours, and then perform conventional freeze-drying. 80% ethanol was used to solidify for 25 minutes, and then conventional freeze-drying was performed again t...
Embodiment 2
[0023] 1. Dissolve 140mg of PLGA (PLA:PLG=50:50) in dichloromethane as the oil phase, dissolve 90ug of bFGF sample in double distilled water as the water phase, then add the bFGF solution into the oil phase and homogenize to form colostrum. Add 40ml of 1% PVA solution, through the action of high-speed milk homogenizer (20000rpm, 2min) to form W / O / W, remove the organic solvent by rotating under reduced pressure with a rotary evaporator, centrifuge to precipitate the microspheres and freeze-dry to obtain the encapsulation PLGA Microspheres of bFGF.
[0024] 2. Add type I collagen to 0.2% acetic acid solution, stir well, and prepare collagen solution.
[0025] 3. Add the microspheres prepared in step 1 to the collagen solution in step 2, stir evenly, add to the mold (or a 96-well plate), pre-freeze at -70°C for 12 hours, and then perform conventional freeze-drying. The collagen sponge carrier embedded with bFGF-PLGA microspheres was obtained by solidifying with 75% ethanol for ...
Embodiment 3
[0027] 1. Dissolve 155mg of PLGA (PLA:PLG=50:50) in dichloromethane as the oil phase, dissolve 100ug of bFGF sample in double distilled water as the water phase, then add the bFGF solution into the oil phase and homogenize to form colostrum. Add 40ml of 1% PVA solution, through the action of high-speed milk homogenizer (20000rpm, 2min) to form W / O / W, remove the organic solvent by rotating under reduced pressure with a rotary evaporator, centrifuge to precipitate the microspheres and freeze-dry to obtain the encapsulation PLGA Microspheres of bFGF.
[0028] 2. Add type I collagen to 0.15% acetic acid solution, stir well, and prepare collagen solution.
[0029] 3. Add the microspheres prepared in step 1 to the collagen solution in step 2, stir evenly, add to the mold (or a 96-well plate), pre-freeze at -70°C for 18 hours, and then perform conventional freeze-drying. The collagen sponge carrier embedded with bFGF-PLGA microspheres was obtained by solidifying with 80% ethanol for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com