Materials for soft and hard tissue repair
a soft and hard tissue and material technology, applied in the field of biomaterials, can solve the problems of high post-operative complication rate of procedures, high risk donor site morbidity, knee instability,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0076]This example describes a biomaterial suitable for tissue repair or augmentation.
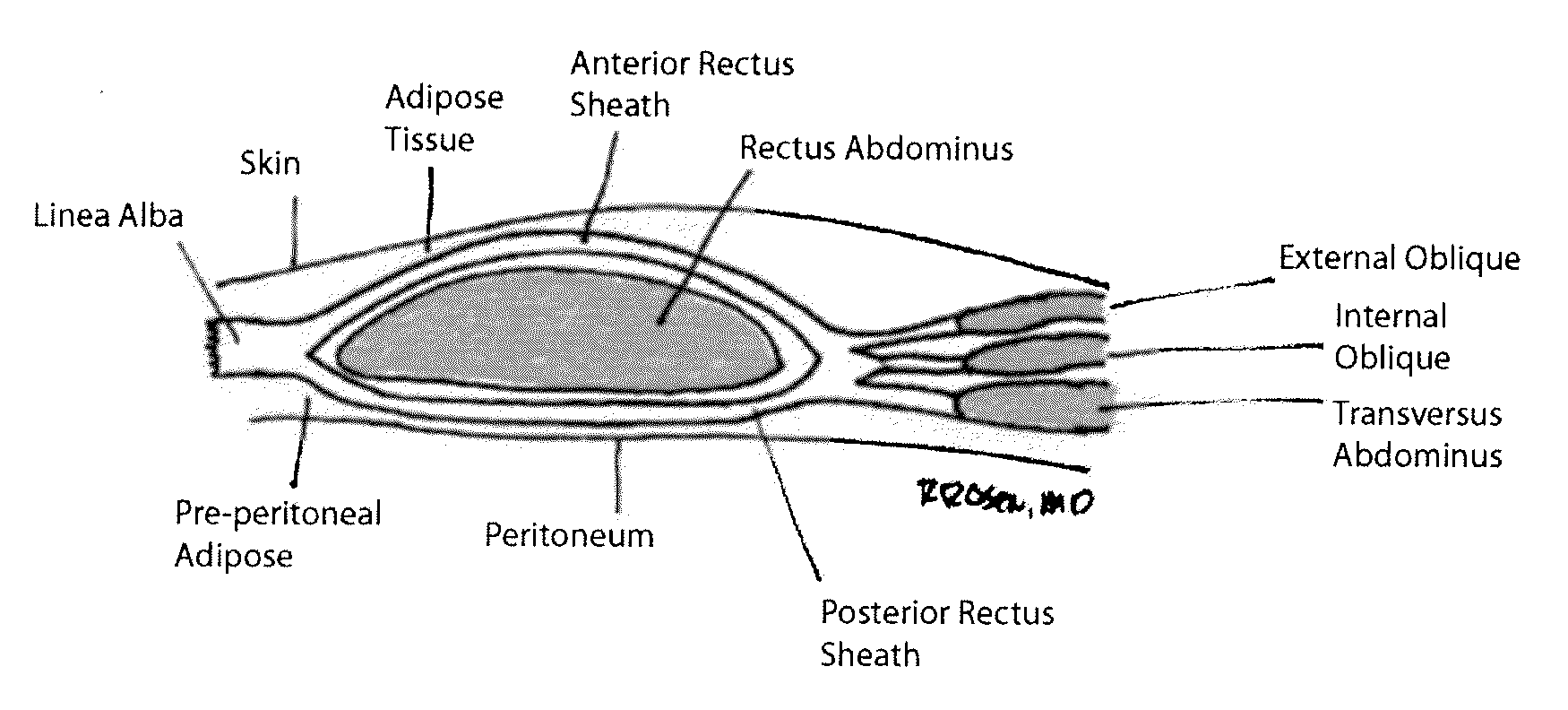
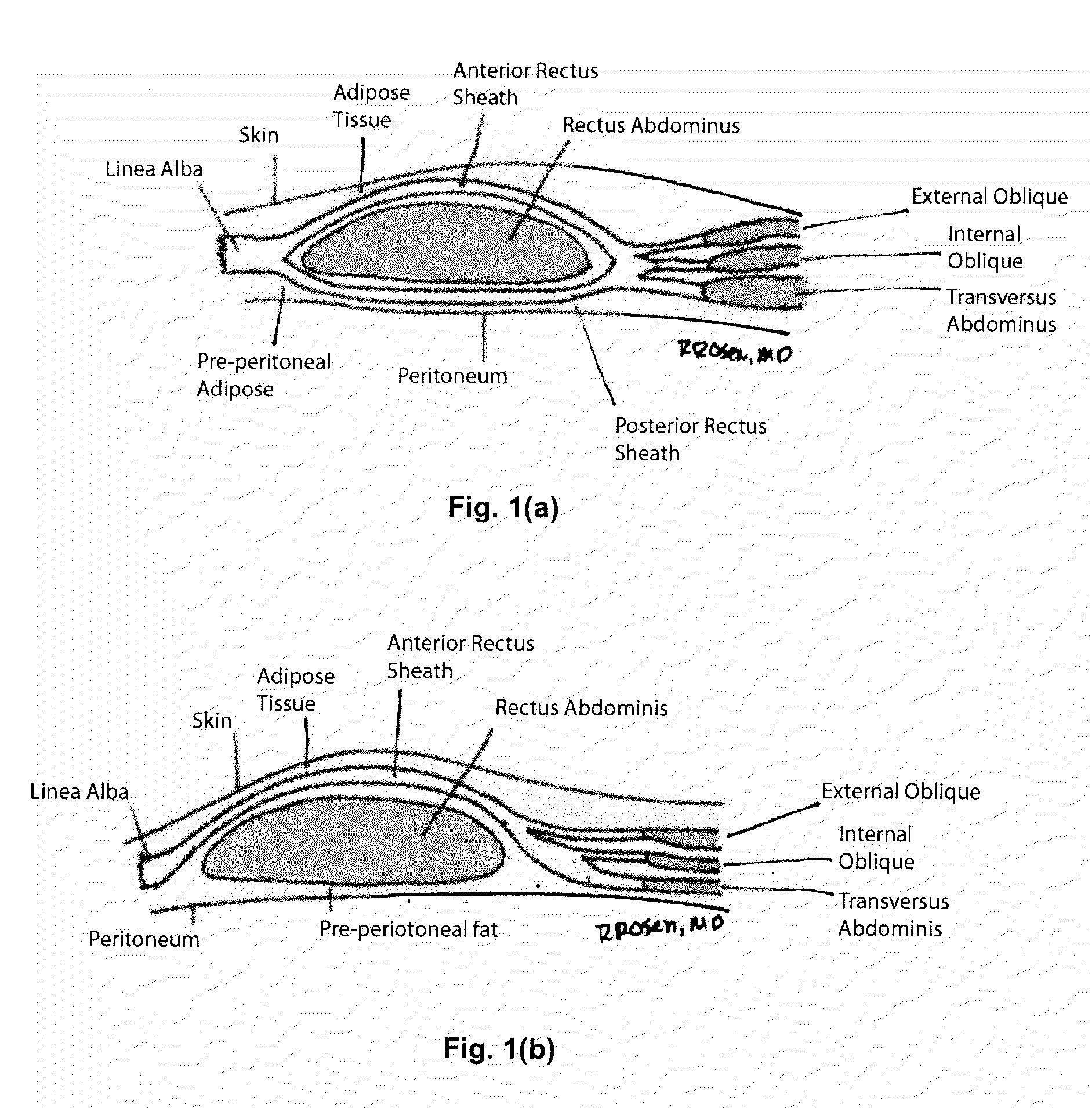
[0077]A section of porcine abdominal wall was harvested from a market-size pig in a USDA inspected facility. The animal was certified for human consumption and further inspected and certified as such by a veterinarian. The entire abdominal wall was harvested by means of a wide, full-thickness circular incision from the costal margins superiorly to the pelvic region inferiorly, harvesting as much tissue as possible and extending posteriorly to the spine. No midline incision was made to preserve the integrity of the rectus sheath. The tissue was transported to an appropriate facility where it was washed thoroughly using room temperature water. The skin and superficial fat was removed by mechanical means using sharp and blunt dissection, and the tissue was washed again with running deionized water. The tissue was disinfected in 0.5% sodium hypochlorite solution, and it was frozen for shipping and stor...
example 2
[0080]This example describes an exemplary biomaterial from bovine shoulder fascia.
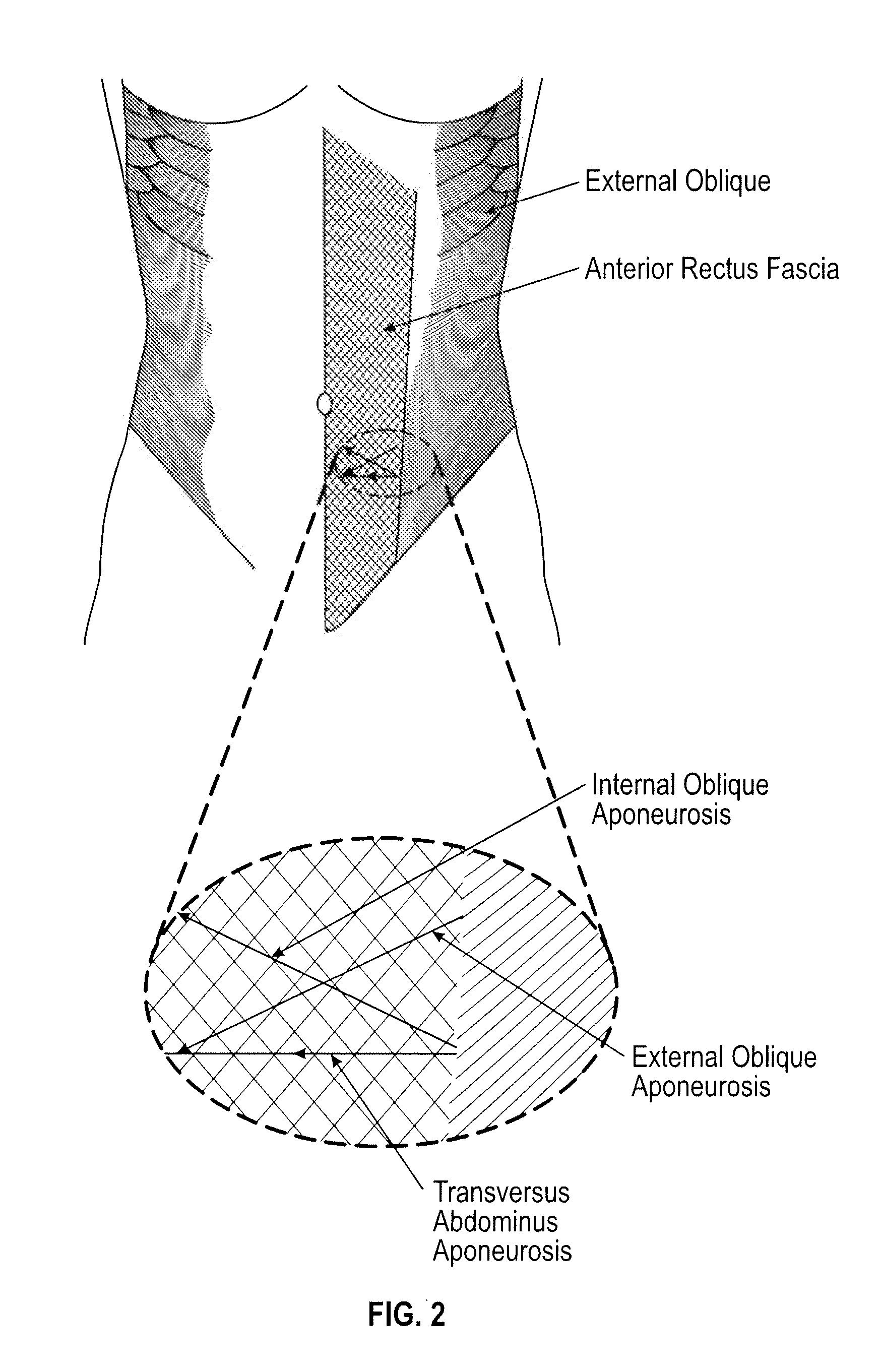
[0081]A section of bovine shoulder fascia was harvested from a market-size calf in a USDA inspected facility. The animal was certified for human consumption and further inspected and certified as such by a veterinarian. The shoulder fascia was harvested by careful dissection of the thick layer of fascia overlying the deltoid, trapezius and omo-brachialis region after the skin and superficial fat are removed. The tissue was disinfected in mild sodium hypochlorite solution and frozen for shipping and storage.
[0082]To begin processing, the tissue was slowly thawed and additional cleaning and dissection of fat and loose connective tissue was carried out until the distinct fascial layers were visualized. Using blunt and sharp dissection, this complex multi-laminar, multi-directional layer was isolated and cleaned, removing as much superficial fat as possible.
[0083]Next, the tissue was rinsed under running, ...
example 3
[0085]This example describes mechanical testing of an exemplary Bovine Fascia Biomaterial.
[0086]The physicomechanical properties of a collagen-based exemplary biomaterial for hernia repair was evaluated using various means of mechanical testings described below to determine the suitability for hernia repair application. These tests were performed as described (Deeken et al., “Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques;” Annals of Surgery, e-publication. Feb. 4, 2012).
[0087]Exemplary scaffolds (biomaterials) were prepared according to the process described in Example 2 herein. Thickness of the biomaterial is indicated in FIG. 7.
A. Laser Micrometry
[0088]The thickness of six scaffolds (biomaterials) was measured using an LK-081 digital laser micrometer and LK-2101 controller (Keyence, Woodcliff Lake, N.J.). The thickness of each scaffold was measured nine times (n=9) and was reported as mean±standard error of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| angles | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com