Recombined bifidobacteria -hRV/VP7 expression vector and oral administration vaccine thereof
A bifidobacteria and expression vector technology, applied in the field of genetic engineering, to achieve the effects of broad clinical application prospects, high safety and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Construction of Bifidobacterium-Escherichia coli shuttle expression vector of the present invention
[0035] 1. Replace the Ptac promoter (183932) on pGEX-5x-1 with the promoter AmyO (position: 599-690nt) of the α-amylase (amylase) gene (GenBank No: AY240946) of bifidobacteria, and simultaneously use PCR Methods The LacIq fragment (position: 3301-4420nt) on pGEX-5x-1 was deleted.
[0036] Specific steps are as follows:
[0037] 1. Use BamHI and SacII enzymes to cut 110 base pairs of the α-amylase gene AmyO (promoter) sequence synthesized by Shanghai Bioengineering Technology Co., Ltd., and add the M13 reverse sequencing primer sequence (taaaacgacggccagt) to its 5' end ( SEQ ID NO.4) for sequencing.
[0038] Its construction route is as follows:
[0039] (1) Promoter sequence of bifidobacterium α-amylase gene: 599 ta aaataaacag catacgttcgcaatagtgca aacgctatca aagaagatga acccccgtta aagggattga agaaaaggaa taaaggagcc690 (SEQ ID NO.5)
[0040] (2) gtaaaacgacgg...
Embodiment 2
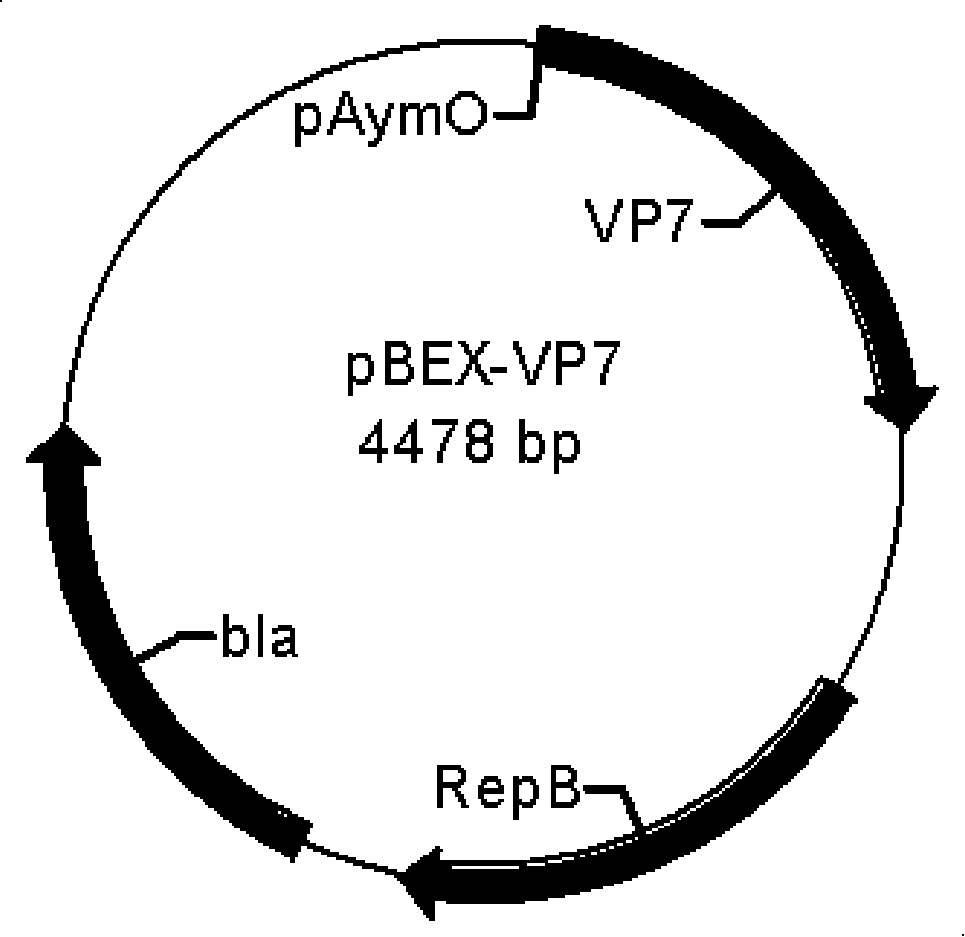
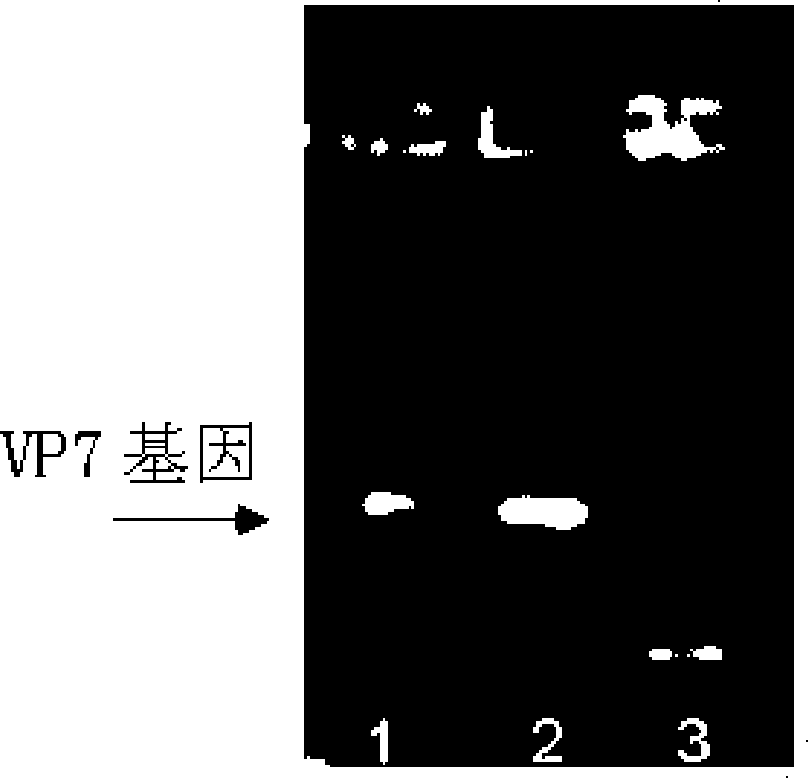
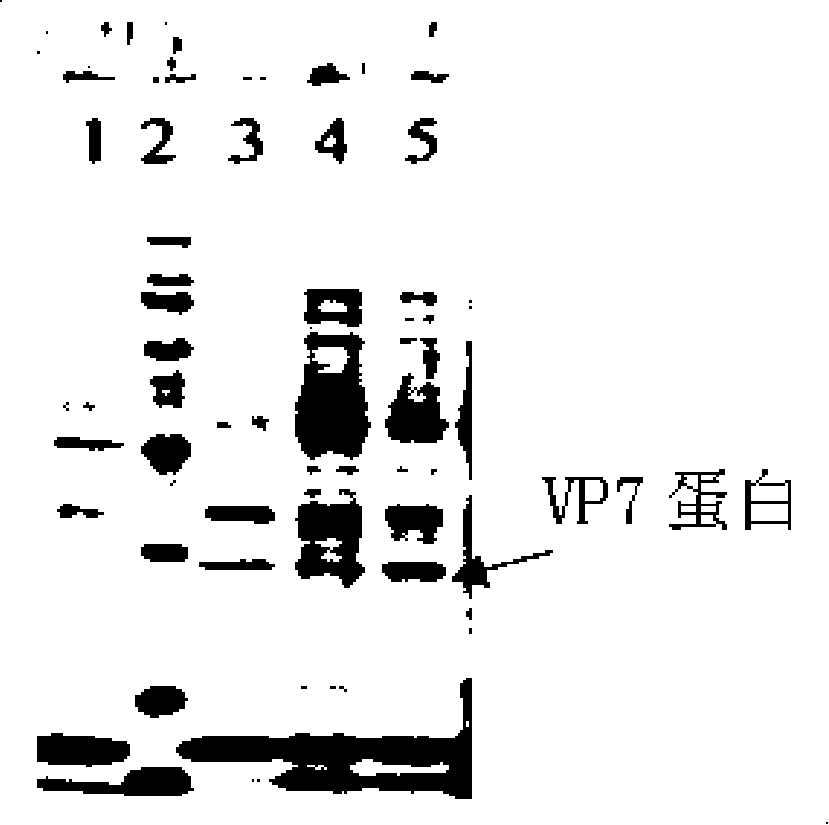
[0063] Example 2 Construction of pBEX-hRV / VP7 expression vector (see attached for a schematic diagram of the construction process Figure 4 )
[0064] Construction of pBEX-hRV / VP7:
[0065] 1, amplify viral protein VP7 gene (GENBANK NO.M21843, VP7, hRV Wa strain, coding region: 49...1029 from the genome of hRVWa strain by RT-PCR method, the VP7 gene used among the present invention is as SEQ shown in ID NO.2).
[0066] Primers are:
[0067] VP7F: atcggatccatgtatggtattgaatatac (SEQ ID NO. 13) (BamHI site added).
[0068] VP7R: acggtcgacctatacctctataataaaaagctg (SEQ ID NO. 14) (SalI site added).
[0069] The method is: take 10 μl of the extracted RNA, heat at 100°C for 2 minutes to denature the double-stranded RNA, and then centrifuge briefly after 2 minutes on ice, then add 4 μl of 5×AMV Bufer, 2 μl of dNTPs, 1 μl of downstream primer (30 μM / μl), RNasin 1μl, reverse transcriptase AMV 2μl, add ddH20 to make up the 25μl reaction system. Reverse transcription at 42°C for 1 h...
Embodiment 3
[0074] Embodiment three The preparation of the bifidobacterium seed bacteria containing pBEX-hRV / VP7 recombinant vector of the present invention
[0075] 1. Screen the bifidobacteria with an OD value of 0.6 as the recipient bacteria. After being treated with sterilized 10% glycerol prepared in deionized water, the pBEX-hRV / VP7 recombinant plasmid vector prepared in Example 2 is transformed into the recipient bacteria by electroporation. In body bacteria. The conversion conditions are: 15KV, 200Ω, 25μF.
[0076] 2. Resuscitate the transformed bifidobacteria in MRS liquid medium without antibiotics for 60-120 minutes, and then culture them on MRS solid medium containing 50 μg / ml ampicillin to select resistant colonies to obtain transformed ETEC For the bifidobacterium with CFA / I gene, the positive clone identified by PCR and SDS-PAGE is the bifidobacterium seed fungus containing the pBEX-hRV / VP7 recombinant vector.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com