A human rotavirus vp8 recombinant protein and a human rotavirus vaccine using the vp8 recombinant protein
A rotavirus and recombinant protein technology, applied in the field of vaccines, can solve the problem of low immunogenicity of protein fragments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Screen foreign peptides and fusion proteins
[0053] Tetanus toxin and diphtheria toxin have been used as human vaccines for nearly 100 years. It is safe to use its fragment polypeptide as a rotavirus vaccine. By analyzing the primary structure of tetanus toxin and diphtheria toxin protein with SwissInstitute Bioinformatics software (http: / / web.expasy.org / compute_pi / ), four polypeptide fragments were screened out (as shown in Table 1). One was from tetanus toxin and three were from diphtheria toxin. These peptide fragments contain 1 to 3 negatively charged amino acids (aspartic acid or glutamic acid) and little or no positively charged amino acids (arginine and lysine), so the isoelectric point of these peptides Both are very low. In this embodiment, the DT4 polypeptide is used as a control. The sequence of the DT4 polypeptide is Gly Val Leu Leu Pro Thr Ile Pro Gly Lys Leu Asp Val Asn Lys Ser Lys ThrHis Ile, and its isoelectric point is 9.7, which contains three posi...
Embodiment 2
[0074] Construction of expression system and protein purification
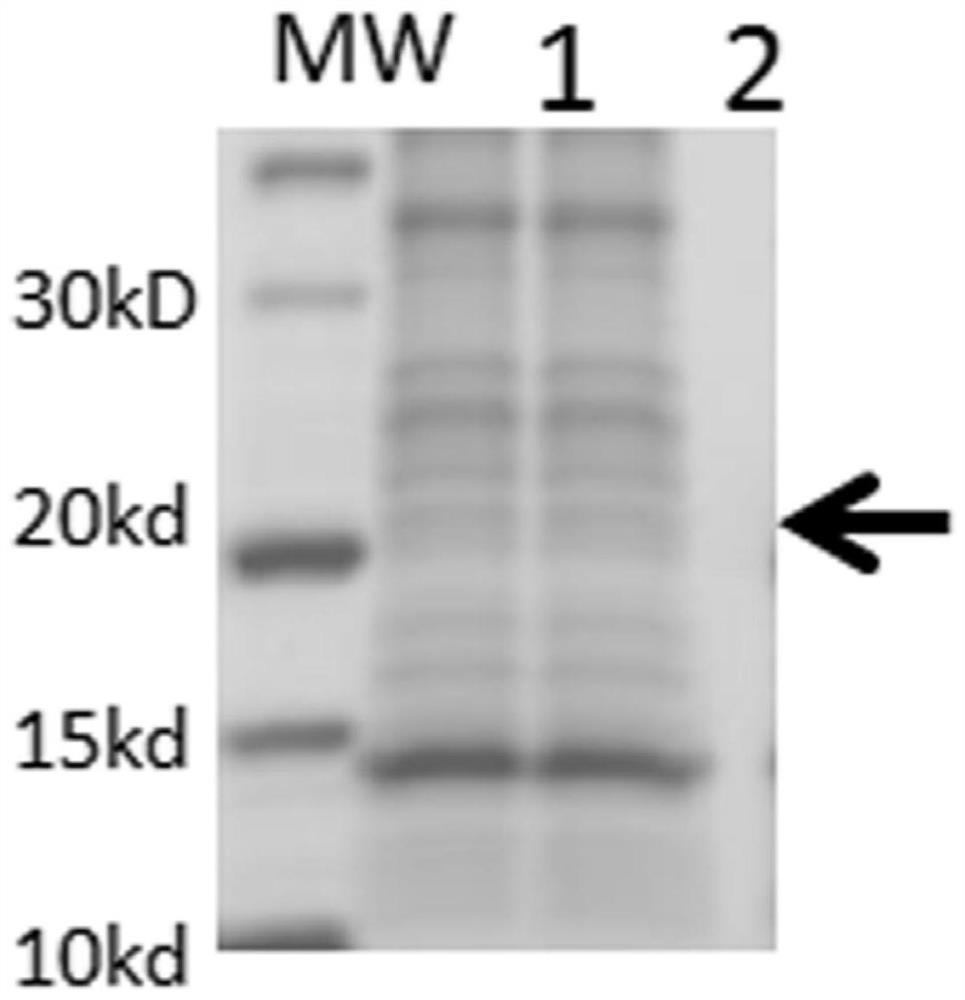
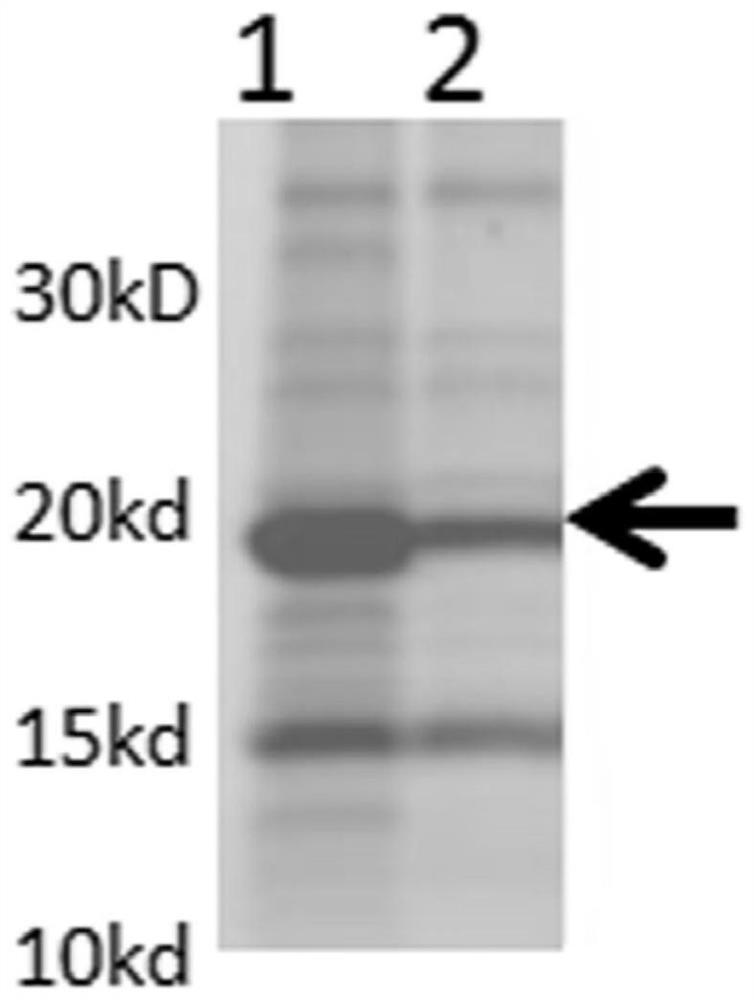
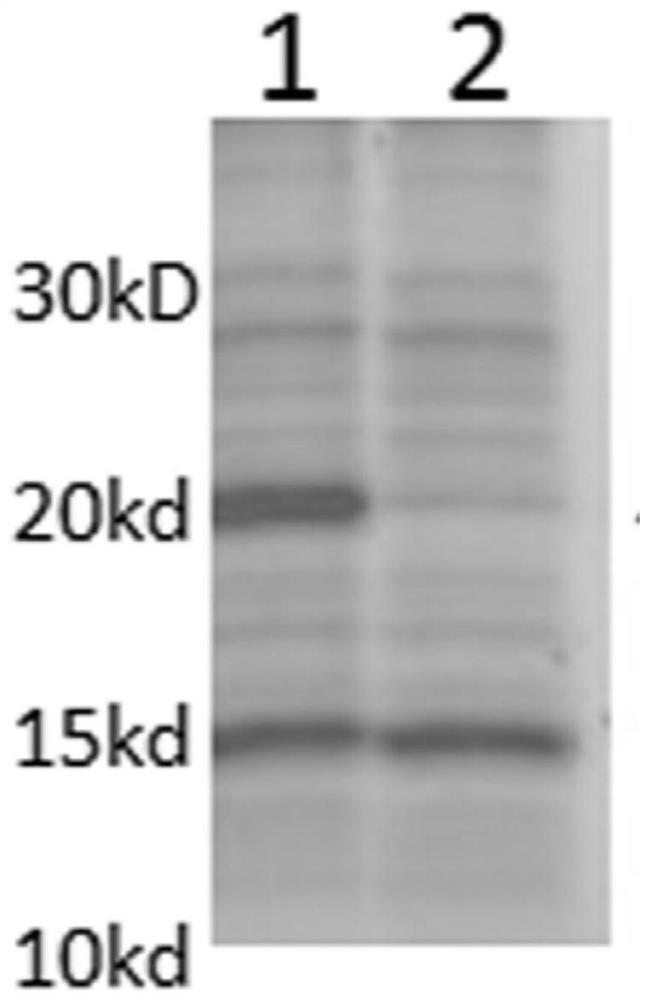
[0075] VP8 protein is a non-glycosylated protein, therefore, the Escherichia coli expression system purchased from Merck Mllipore was selected . The plasmid used was pET30a ; host cell E. coli BL21(DE3). Firstly, codon optimization was carried out on the DNA sequences of the above six proteins, and then their artificially synthesized nucleotide sequences ( http: / / www.blueheronbio.com / ), and cloned into pET30a plasmid expression system. Then transform the plasmid with the target gene into BL21(DE3) competent bacteria for antibiotic selection. Confirm that the well-grown colonies were picked and cultured in a shaker flask at 37°C until the turbidity was 1-1.5 (OD600), then the culture temperature was lowered to 20°C, and 2mM IPTG was added to induce expression for 4-6 hours. Then the bacterial cells were collected, lysed by high pressure (ATS lyser), and centrifuged. Samples were taken before and after c...
Embodiment 3
[0081]Production of monoclonal antibodies. In this example, VP8P[8] protein was used to immunize mice (BALB / c) to produce monoclonal antibodies. 20 micrograms of VP8P[8] adsorbed on 100 micrograms of aluminum hydroxide were used to subcutaneously inject mice 3 times with an interval of 7 days. Blood was collected on the 21st day, and an enzyme-linked immunoassay test found that there was a high titer of anti-VP8P[8] protein antibody in the serum (1:105 dilution positive). Splenocytes from a mouse were cultured in fusion with myeloma cells, and 22 monoclonal antibodies were screened by VP8P[8] protein-coated ELISA. Then use the fluorescent labeling method to further detect whether these monoclonal antibodies can bind to the VP8 protein on the surface of the virus. In a 6-well cell culture dish, grown on top of a 100% MA104 cell monolayer, approximately 100x CC ID50 of JX406750 virus was added. After culturing at 37 degrees for 18 hours, add monoclonal antibody culture soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com