Multivalent huma-bovine retavirus vaccine
A technology of bovine rotavirus and rotavirus, applied in the field of multivalent human-bovine rotavirus vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
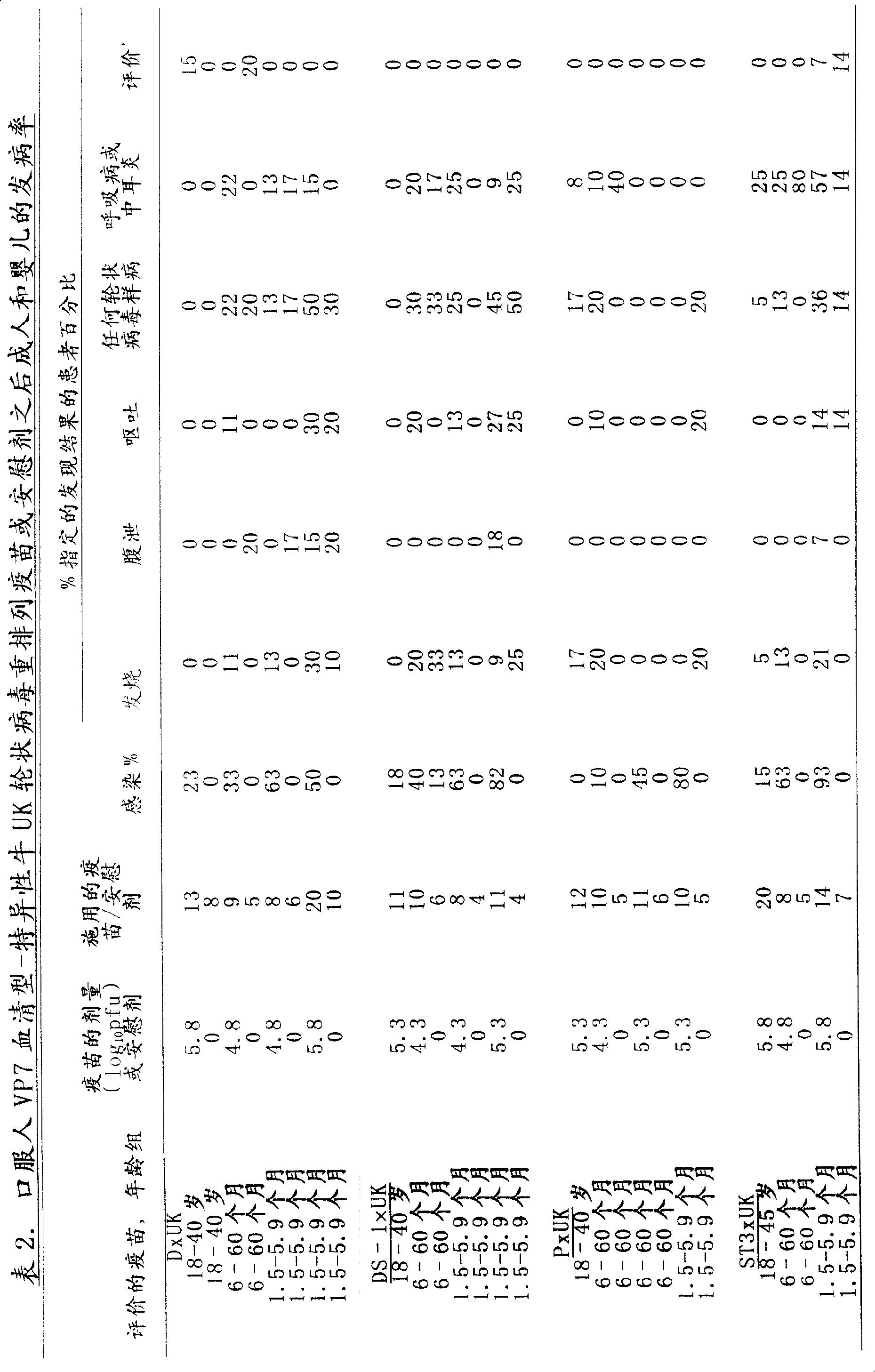
[0038] This example describes rotaviruses derived from human rotavirus strains D (VP7:1), DS-1 (VP7:2), P (VP7:3) and ST (VP7:4) and bovine UK Compton (UK) rotaviruses. The rotavirus rearrangements were prepared, and the safety, immunogenicity and reactogenicity of each rearrangement were evaluated for adults, children and infants.
[0039] From bovine UK Compton (UK) strains and from human rotavirus strains D (VP7 serotype 1, ATCC VR-970), DS-1 (VP7 serotype 2; Wyatt et al., Perspect. Virol. 10:121-145 (1978)) and P (VP7 serotype 3, Wyatt et al., Science 207:189-171 (1983)) and ST3 (VP7 serotype 4; Banatvala et al., Journal of the American Veterinary Medical Association (J.Am. Vet. Med. Assoc.) 173:527-530 (1978)) derived human X bovine rearranged rotavirus strains representing VP7 serotypes 1, 2, 3 and 4. Human rotavirus strains D, DS-1 and P were recovered from the stool of children hospitalized with diarrhea; strains D and DS-1 were propagated and passaged in sterile calv...
Embodiment II
[0074] This example describes a tetravalent human x bovine rearranged rotavirus immunogenic composition evaluated for its clinical safety and immunogenicity in adults, children and infants.
[0075] The four human x bovine rearranged rotaviruses described in Example 1 were mixed in equal volumes to form a single tetravalent vaccine composition. All studies were performed in a placebo-controlled fashion to evaluate the safety and immunogenicity of the mixed compositions. All serotyping and microbiological determinations were performed as described in Example 1.
[0076] A single dose of undiluted 10 5.3 -10 5.8PFU per rearranged quadrivalent human (VP7 serotypes 1, 2, 3 and 4)-bovine UK rotavirus vaccine. Study subjects fasted for at least 1 hour before or after administration of the vaccine or placebo. They were given 120 ml of a buffer (sodium bicarbonate) to neutralize stomach acid, followed one minute later by either the tetravalent immunogenic composition mixed with th...
Embodiment III
[0092] This example provides data from an ongoing clinical trial comparing the preferred tetravalent human-bovine rearranged rotavirus composition of the invention with the approved tetravalent macaque-human rotavirus rearranged vaccine ROTASHIELD. Summary of preliminary phase analysis. This assay examined the proportion of low-level febrile responses and protective efficacy against rotavirus diarrhea for both compositions.
[0093] A phased two-year clinical study is currently underway to compare the quadrivalent human-bovine rearranged rotavirus composition of the present invention with the quadrivalent macaque-human rotavirus rearranged vaccine (recently approved in 15 countries in the United States and the European Community) Safety and efficacy of anti-rotavirus diarrhea using ROTASHIELD). The study was conducted in Finland and included 172 quadrivalent human-bovine rotavirus rearrangement subjects, 86 corresponding placebo controls, 161 ROTASHIELD vaccinators and 79 cor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com