Serogroup B meningococcus recombinant chimeric protein vaccine and preparation method thereof
A chimeric protein, meningococcal meningitis technology, applied in the field of group B meningococcal recombinant chimeric protein vaccine and its preparation, can solve the problem of lack of immunogenicity, achieve effective vaccine protection effect, increase preventive effect, Enhanced immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1P2-f
[0056] The construction of embodiment 1P2-fHBP V1 engineering strain:
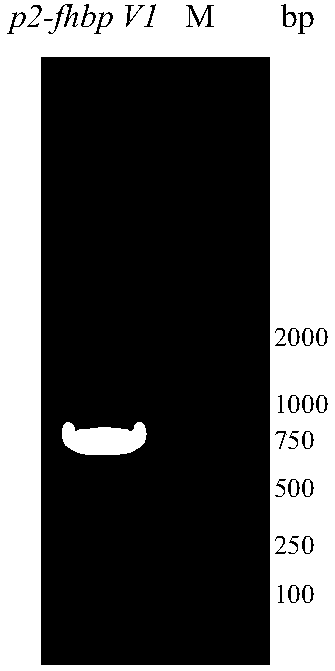
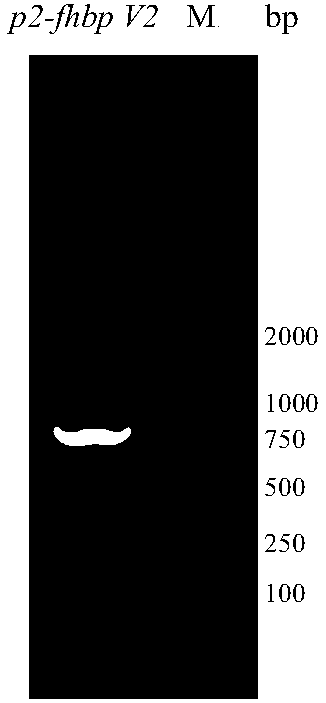
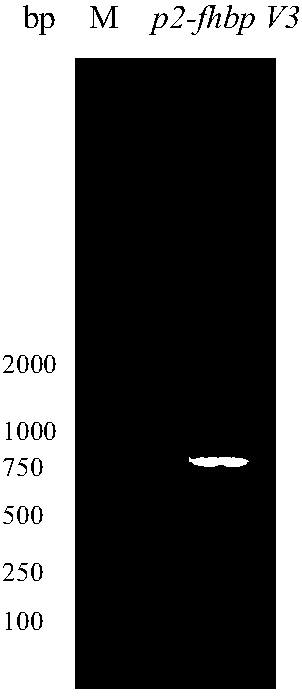
[0057] According to the amino acid sequence of fHBP V1 and P2 and the codon preference of Escherichia coli, the full-length DNA sequence p2-fhbp V1 was designed and synthesized, and p2 was connected to the N-terminus of fhbp V1 as a gene synthesis plasmid, and the gene p2-fhbp V1 was synthesized into plasmid pUC57 Above, the gene synthesis plasmid pUC57 and the vector pET43.1a were digested with restriction enzymes NdeI and XhoI to obtain the target gene fragment and the digested plasmid pET43.1a; the target gene fragment p2-fhbp V1 was combined with the plasmid pET43. 1a was ligated with T4ligase, and the ligated product was transformed into E.coli DH5α, and cultured overnight at 37°C. Pick positive clones to inoculate LB liquid medium, shake culture at 37°C overnight. The target gene fragment was amplified by PCR method, and the analysis and identification results were as follows: figure 1 It is shown ...
Embodiment 2P2-f
[0059] The fermentation of embodiment 2P2-fHBP V1 engineering bacteria
[0060] The strains were inoculated into LB liquid medium, and cultured at 37° C. and 200 rpm for 12 hours. Expand the inoculation to the fermenter at a ratio of 5%, culture at 37°C for 4-8 hours, and when the OD600 increases to 18-22, induce with IPTG at a final concentration of 0.05mM for 4-8 hours. Bacteria were collected by centrifugation in a large-capacity centrifuge.
Embodiment 3P2-f
[0061] Extraction and purification of embodiment 3P2-fHBP V1 recombinant chimeric protein
[0062] Bacterial destruction: Take the bacterial cells and add the bacterial destruction buffer (20mM PB, 2mM EDTA, pH7.0), homogeneously destroy the bacteria twice at low temperature (4°C) and ultra-high pressure (1100-1400bar), then centrifuge at a high speed (10000rpm, 60min , 4°C) to take the broken supernatant of bacteria.
[0063] Protein extraction: using the method of ammonium sulfate fractional precipitation, 15% ammonium sulfate was precipitated at 4°C for 1 hour, centrifuged at 6500rpm at 8°C for 60 minutes, and the centrifuged supernatant was taken; the supernatant was stirred and precipitated with 45% ammonium sulfate at 4°C for 1 hour, The protein precipitate was dissolved in 20mM PB pH7.4, ultrafiltered with a 10KD membrane bag and concentrated to 1 / 5-1 / 3 of the original volume.
[0064] Refining protein purification: Sepharose QFF was used to load the sample with 20mM P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com