Ror1(ntrkr1)specific chimeric antigen receptors for cancer immunotherapy
A chimeric antigen receptor and specific technology, applied in the direction of targeting specific cell fusion, medical raw materials derived from mammals, antibodies, etc., can solve the problem of increasing the complexity of initial risk stratification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0371] Example 1: Screening of ROR1 positive and negative cell lines
[0372] To identify cell lines expressing different cell surface expression levels of ROR1, 12 human cell lines were analyzed by flow cytometry using Qifikit (Dako) and anti-human ROR1 mAb clone 2A2 (see Materials and Methods). Nine of these cell lines have been previously described in the literature to be positive for ROR1 at the mRNA or protein level (Table 10).
[0373] Table 10: Cell lines positive for ROR1 reported in the literature
[0374] cell line describe cell type MDA-MB-231 Adhesive breast cancer PC-3 Adhesive Prostate adenocarcinoma MDA-MB-468 Adhesive breast cancer Hs 746T Adhesive stomach cancer NCI-H1993 Adhesive non-small cell lung cancer HT-29 Adhesive colorectal adenocarcinoma A549 Adhesive human lung adenocarcinoma Cal51 Adhesive breast cancer Jeko-1 floating mantle cell lymphoma
[0375] Fl...
Embodiment 2
[0379] Example 2: Production of anti-ROR1 scCAR
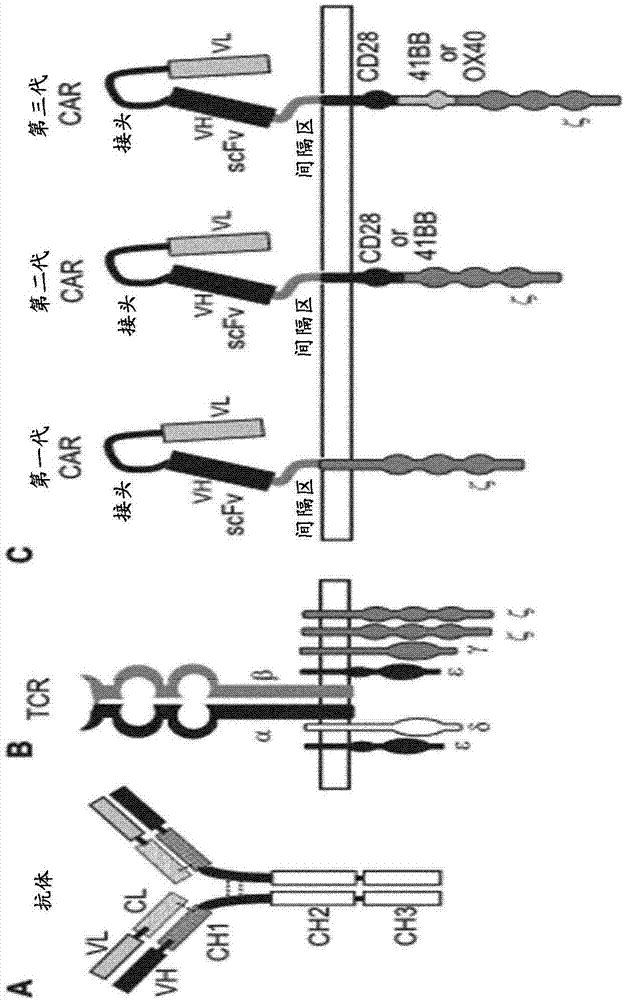
[0380] A panel of 18 second-generation ROR1-specific scCARs was generated and tested in the following experiments ( figure 1 C).
[0381] They are produced by the fusion of the building blocks of Table 1-2 below:
[0382] - a scFv from murine origin or a humanized form from D10, G6, G3, H10, 2A4 or 1C11;
[0383] - a spacer: human FcγRIIIα, CD8α or IgG1 hinge;
[0384] - the transmembrane domain of human CD8α and the co-stimulatory domain of human 41BB;
[0385] - Activation domain of human CD3ζ
[0386] Different scFvs are used in scCARs to generate receptors with different binding affinities and different epitope specificities.
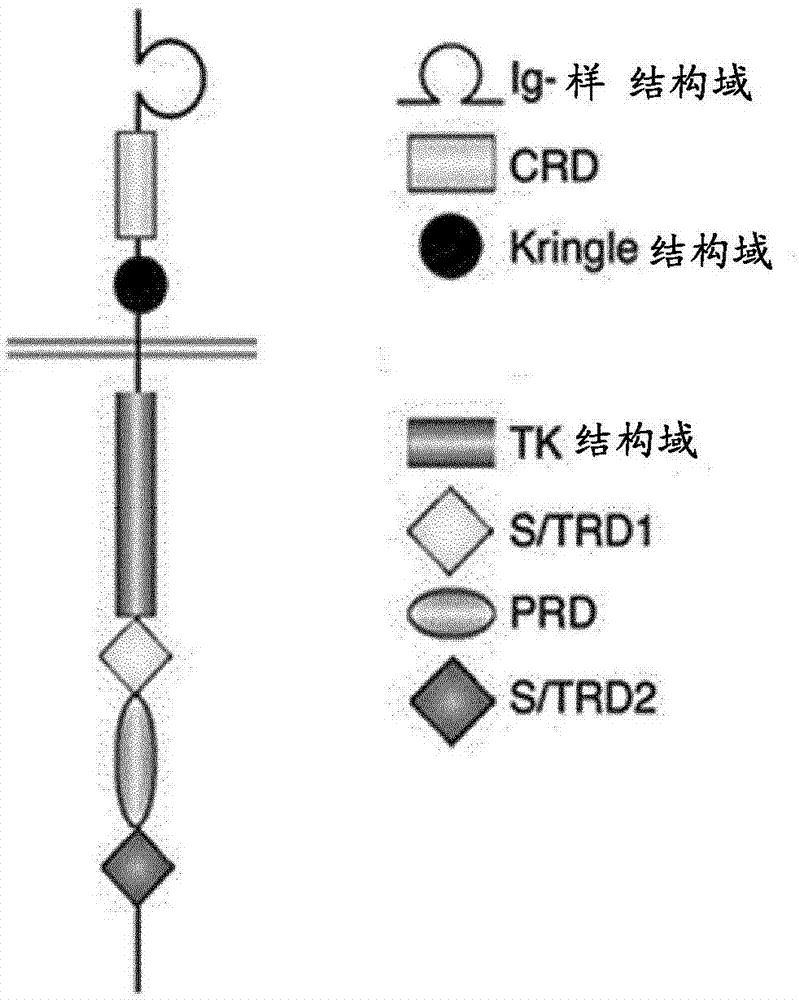
[0387] mAbs D10 and G6 target the 3' Ig-like region and the linker region between the Ig-like domain and the CRD domain of ROR1 ( figure 2 )
[0388] mAbs G3, H10 and 2A4 target the IgG-like region of ROR1 ( figure 2 )
[0389] mAb 1C11 targets the CRD domain of ROR1 ( figure 2 ).
[0390...
Embodiment 3
[0391] Example 3: In vitro testing of anti-ROR1 scCAR
[0392] In human primary T cells, use the Figure 6 The two-step screening presented in tested previously designed anti-ROR1 scCARs.
[0393] In the first step of the screening method described, primary human T cells preactivated for 4-5 days with anti-CD28 / CD3 beads and IL-2 were treated with mRNAs encoding the 18 anti-ROR1 scCARs presented earlier. Cell electroporation. One day after electroporation, scCAR expression was assessed by flow cytometry and western blotting, and scCAR-modified T cell activity was assessed by measuring T cell degranulation. ScCARs detected by Western blot and that induced significant specific T cell degranulation (≥20%) when co-cultured with at least one ROR1-positive cell line were selected to undergo the second step of the screening method.
[0394] In the second step of the screening method, primary human T cells previously activated for 11-12 days with anti-CD28 / CD3 beads and IL-2 were t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com