Multi-component group B meningococcus vaccine and preparation method thereof
A meningococcal and multi-component technology is applied in the field of multi-component B meningococcal vaccine and its preparation, and can solve the problems of lack of bactericidal power and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
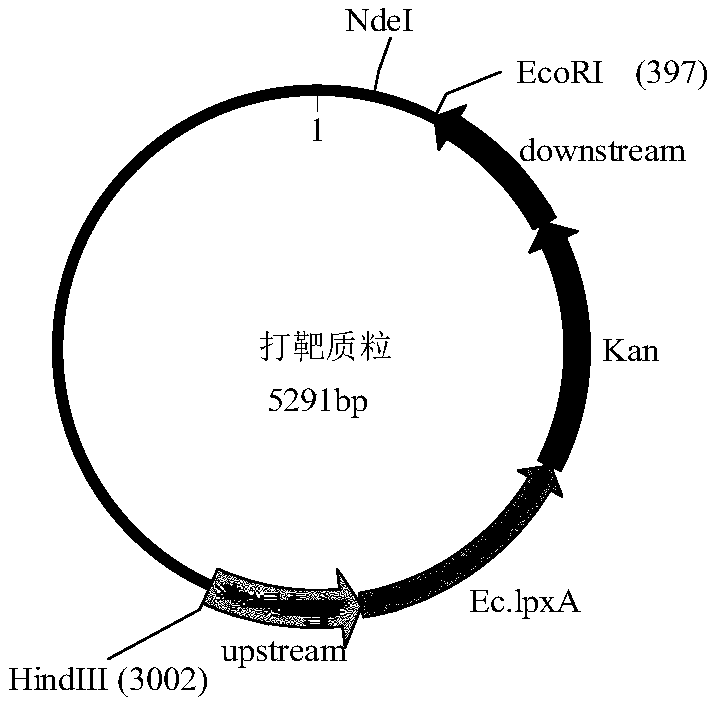
[0081] Embodiment 1: Construction of group B meningococcal low endotoxin mutant strain:
[0082] In this embodiment, the ST4821 clone group 341215 strain of meningococcus B epidemic strain is taken as an example.
[0083] 1.1 According to the lpxA gene sequence of group B meningococcus, design and synthesize primers as follows:
[0084] 5' TGATTACGCCAAGCTTCAAAAACTCATCCCCCCA 3'
[0085] 5'TATCAATCACGTTTTTCCTTTTCCTGTCG 3'
[0086] Use the primers to PCR amplify the upstream 500bp sequence of the lpxA gene of group B meningococcal strain 341215;
[0087] 1.2 According to the lpxA gene sequence of Escherichia coli DH5α strain, design synthetic primers as follows:
[0088] 5'AAGGAAAAACGTGATTGATAAATCCGCC 3'
[0089] 5'TATGGCTCATTTAACGAATCAGACCGCG 3'
[0090] Using the primers to PCR amplify the lpxA gene of Escherichia coli DH5α strain;
[0091] 1.3 According to the kanamycin resistance gene sequence on the pET28a plasmid, design synthetic primers as follows:
[0092] 5'GATTC...
Embodiment 2
[0107] Example 2: Extraction of group B meningococcal low endotoxin mutant strain OMV
[0108] 2.1 Cultivation of group B meningococcal low endotoxin mutant strains
[0109] Spread and inoculate the low-endotoxin mutant strain of group B meningococcus frozen at -70°C on 10% sheep blood ordinary agar medium, at 37°C, 5% CO 2 Incubate overnight. Scrape the bacterial lawn and inoculate it into the nutrient broth medium, cultivate it at 37°C and 180rpm for 4-6 hours, and gradually expand the culture in 30L, 100L, and 500L fermenters until the OD value is about 6-12, and add a final concentration of 0.01-0.1ug / ml of sodium deoxycholate solution sterilized for 5-20 minutes.
[0110] 2.2 Extraction of group B meningococcal low endotoxin mutant strain OMV
[0111] Take the fermented bacteria liquid and collect the bacteria by centrifugation in a disc centrifuge, suspend the bacteria in 50mM PB, 1mM Na2·EDTApH7.2 solution 5-10 times, and then homogeneously crush 2-3 times at 1000-1...
Embodiment 3
[0112] Embodiment 3: Preparation of fHbp-A recombinant lipoprotein
[0113] 3.1 Construction of fHbp-A / BL21(DE3) engineering bacteria
[0114] According to the amino acid sequence of fHbp-A, Haemophilus influenzae P4 outer membrane protein signal peptide and the codon preference of Escherichia coli, the P4 signal peptide was directly located at the N-terminus of fHbp-A, and the full-length DNA sequence was designed and synthesized on plasmid pUC57;
[0115] The pUC57 synthetic plasmid was treated with restriction endonucleases NdeI and XhoI to obtain a large fragment of (p4)fhbp-A gene.
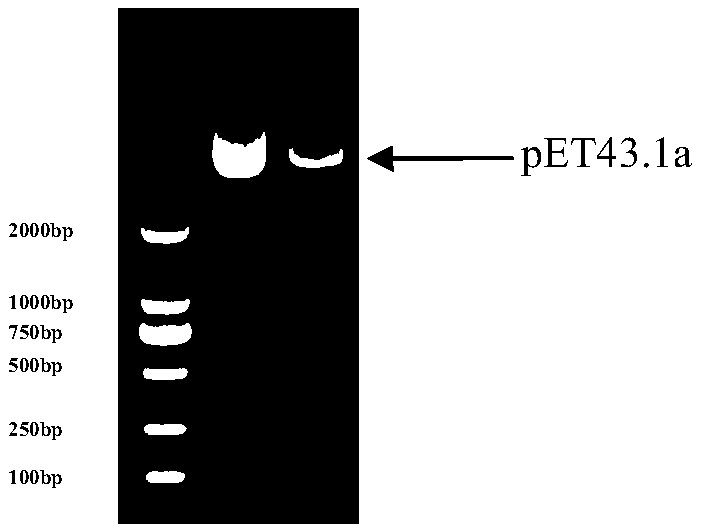
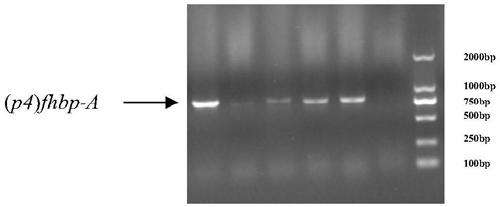
[0116] Treat the pET43.1a plasmid with restriction endonucleases NdeI and XhoI to obtain the digested plasmid pET43.1a, such as figure 2 .
[0117] The treated target gene fragment (P4) fHbp-A and plasmid pET43.1a were ligated with a recombinase, and the ligated product was transformed into E.coli DH5α, and cultured overnight at 37°C. Positive clones were picked and inoculated in LB (Amp) l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com