Monoclonal antibody capable of resisting group A meningococcal capsular polysaccharide conjugate, hybridoma cell strain and applications
A hybridoma cell line, meningococcal technology, applied in the direction of anti-bacterial immunoglobulin, microorganisms, immunoglobulin, etc., can solve the problems that need to be verified, and achieve the effect of high antibody titer, simple detection process, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Establishment of anti-group A Neisseria meningitidis polysaccharide monoclonal antibody hybridoma cell line WV-A-01
[0035] Unless otherwise specified, the vaccines and polysaccharides involved in this example can be prepared according to Part Three of the Pharmacopoeia of the People's Republic of China 2015 Edition or obtained from the market.
[0036] 1.1 Source of antigen
[0037] 1.1.1 Group A meningococcal diphtheria toxoid non-toxic variant polysaccharide conjugate stock solution (batch number MenAps-CRM 197 20140201), provided by the Technology Center of Yunnan Watson Biotechnology Co., Ltd.;
[0038] 1.2 Source of mice
[0039] Female, 6-8-week-old BALB / c mice, SPF grade (Specific pathogen Free, SPF), 15-20 g, were purchased from Guangdong Medical Experimental Animal Center (permit number SCXK (Guangdong) 20130002).
[0040] 1.3 Source and maintenance of myeloma cells
[0041] Myeloma cells SP2 / 0 were purchased from the Kunming Cell Bank of the T...
Embodiment 2
[0082] Embodiment 2: Preparation of monoclonal antibody (mouse ascites)
[0083] 2.1 Pretreatment of mice
[0084] Three BALB / c mice aged 8 to 10 weeks were injected with sterile liquid paraffin, 0.5ml / mouse, and used to inoculate hybridoma cells 7 days later.
[0085] 2.2 Recovery, subcloning and expansion of hybridoma cells
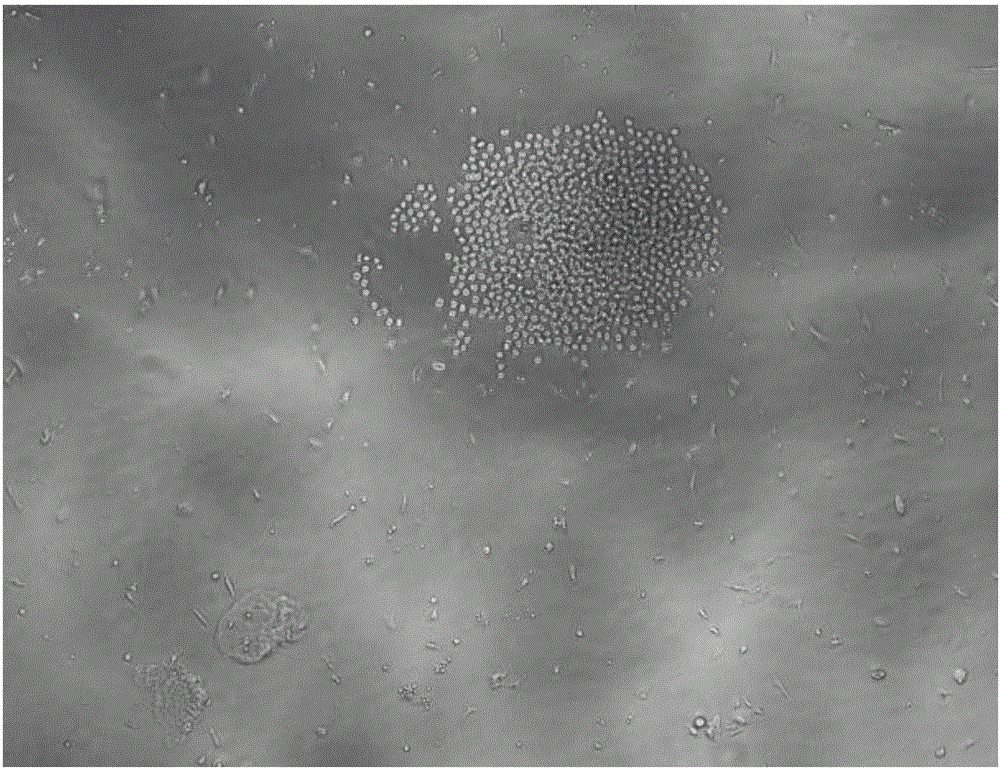
[0086] Resuscitation was carried out according to the method in 1.6. During the culture process, pay attention to observe the number and shape of cells. Subcloning was carried out according to the limited dilution method. %, combined with observation under a microscope, select cells with better morphology for expanded culture (see figure 1 , figure 2 ).
[0087] 2.3 Mice were inoculated with hybridoma cells
[0088] When the cells cover 80% to 90% of the bottom of the plate, blow the cells down, centrifuge at 1200r / min for 5min, discard the supernatant, suspend the cells with RPMI-1640 medium, count them, and adjust the number of cells to 5×10 5 ...
Embodiment 3
[0091] Example 3: Purification of monoclonal antibodies
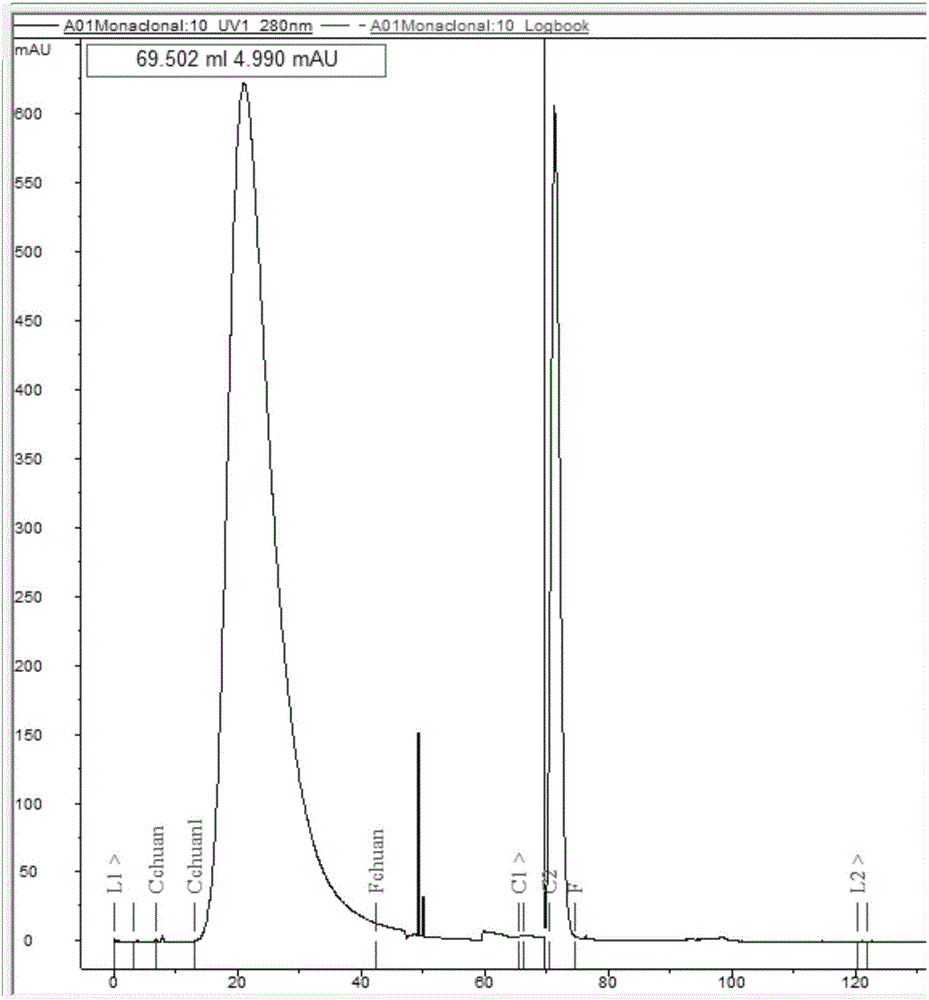
[0092] 3.1 Use Protein A-Sepharose CL-4B (filler) affinity chromatography to purify the monoclonal antibody, monitor with AKTAexplorer100 (protein purification system), see the purification chromatography image 3 , the specific operation is as follows:
[0093] 3.1.1 Column packing: Dissolve 1.5g Protein A-Sepharose CL-4B dry powder in 6-7ml distilled water, soak in 0.02M, pH7.4 phosphate buffer for 15min, and then load it into the chromatography column.
[0094] 3.1.2 Equilibrium: pass through the column with 10 times of the column bed volume of the sample buffer, the flow rate is 1ml / min, and the pH of the effluent liquid tested by pH test paper is 7.4.
[0095] 3.1.3 Sample loading: Take 5ml of pretreated ascites, dilute to 50ml with loading buffer, filter with a 0.45μm filter membrane, and load the sample at a flow rate of 1ml / min.
[0096] 3.1.4 Elution: Wash with loading buffer, 10 times the column bed volume, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com