Multivalent group b meningococcal protein vaccine and preparation method thereof
A meningococcal and protein vaccine technology, applied in the preparation methods of peptides, chemical instruments and methods, bacteria, etc., can solve the complexity of the vaccine preparation process, the excessive purification, the difficulty of 4CMenB vaccine to achieve wide coverage, the change of three-dimensional natural conformation, etc. problems, to achieve good protection and immunity persistence, to ensure vaccine protection coverage, and to maintain the effect of three-dimensional natural conformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Preparation method of multivalent group B meningococcal protein vaccine
[0074] The serotypes, subtypes, and origins of the four serogroup B meningococcal strains are as follows:
[0075] CMCC29356 strain, serotype 4, subtype: P1.19,15 (B4,P1.19,15), from the China Medical Bacteria Collection Center (CMCC); provided by the Netherlands National Institute of Public Health and Environmental Protection.
[0076] CMCC29361 strain, serotype 15, subtype: P1.7, 16 (B15, P1.7, 16), from the China Medical Bacteria Collection Center (CMCC); provided by the Netherlands National Institute of Public Health and Environmental Protection.
[0077] xrsw341215 strain, serotype 3, subtype: P1.7-2, 14, ST-4821 (B3, P1.7-24, ST-4821), isolated from a blood sample of a domestic group B epidemic cerebrospinal meningitis patient , deposit number CGMCC No.8982.
[0078] Strain xrsw210902, serotype 3, subtype: P1.22, 26, ST-8919 (B3, P1.22, 26, ST-8919), isolated from domestic cerebr...
Embodiment 2
[0131] Example 2 Determination of the cross-antibody titer of group B meningococcal vaccine strains to dominant pathogenic strains at home and abroad
[0132] 1. Source of the strain
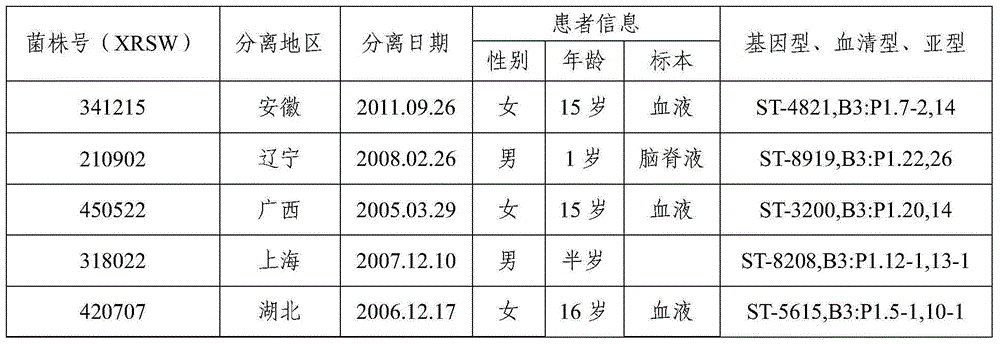
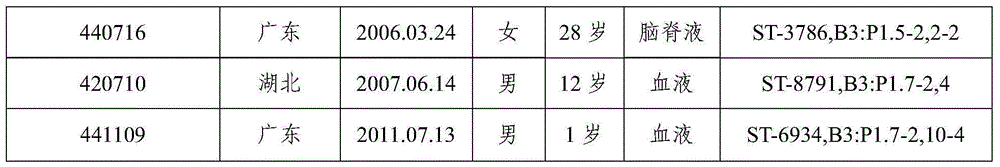
[0133] 1.1 Domestic group B strains: Screened from 167 domestically collected group B meningococcal meningitis patients and carriers, and the identified patients isolated dominant pathogenic strains, a total of 8 strains (Table 1).
[0134] Table 1 Predominant pathogenic strains of domestic group B meningitis patients
[0135]
[0136]
[0137] 1.2 Group B strains from foreign countries come from the US FDA, the Netherlands National Institute of Public Health and Environmental Protection, and the China National Institutes for Food and Drug Control, with a total of 7 strains (Table 2).
[0138] Table 2 Foreign strains and dominant pathogenic strains of Group B vaccines
[0139]
[0140] 1. Group 3A meningococcal strains (Table 3).
[0141] Table 3 Three strains of group A meningococca...
Embodiment 3
[0167] Example 3 Acute Toxicity and Abnormal Toxicity Tests
[0168] This test utilizes the acute toxicity reaction of medicine of different doses, injects the test animal (mice, guinea pig) body of certain dose of need test solution (multivalent B group meningococcal protein vaccine prepared in embodiment 1), in the stipulated Observe the toxic reaction symptoms and death of animals within a certain period of time, and judge whether the test product meets the specified quality requirements and its safety degree.
[0169] 1. Experimental method:
[0170] 1.1 Experimental animals
[0171] Mice: NIH mice, body weight: 18-22 g / mouse, 5 in each group;
[0172] Guinea pigs: body weight: 250-350 g / guinea pig, 2 guinea pigs per group;
[0173] 1.2 Injection dose and grouping
[0174] 1.2.1 Abnormal toxicity test: According to the inoculation dose stipulated in the abnormal toxicity inspection method of the three appendix XIIF items of the 2010 edition of "Chinese Pharmacopoeia": ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com