Meningococcal vaccine and its preparing method
A meningococcal and combined vaccine technology, which is applied in chemical instruments and methods, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of high equipment investment requirements, no further separation, and numerous and complicated steps, so as to improve immunogenicity , Reduction of reactogenicity, effect of purification process improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of Group B Meningococcal Outer Membrane Protein
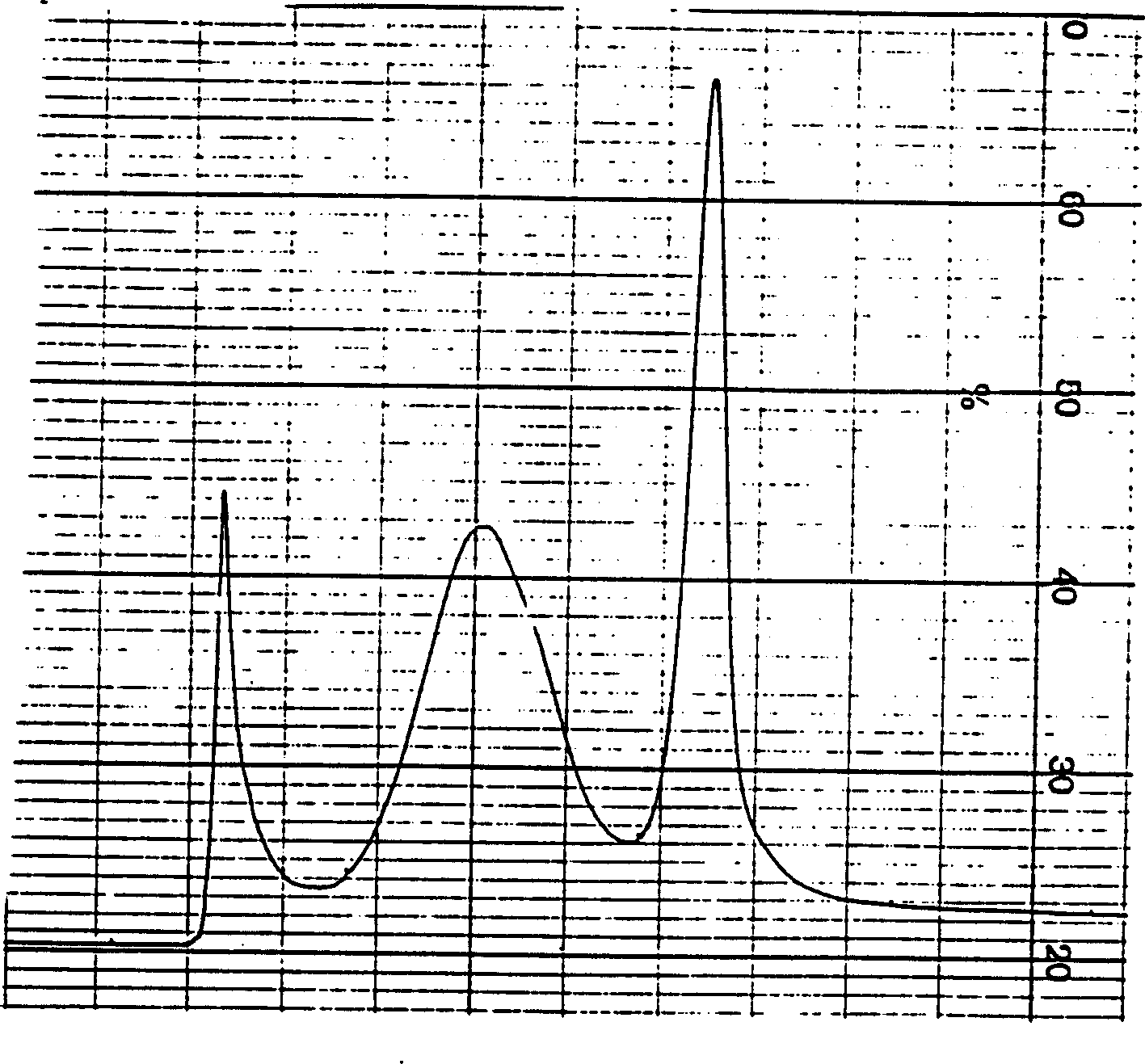
[0050] Kaikai Group B meningococcal strains were inoculated on a semi-comprehensive slope, expanded and transferred to the fifth-generation liquid tank for culture, and the bacteria were collected by continuous centrifugation, and 0.3-0.6M LiCl and 0.2-0.5MCH 3 The COONa mixture was used to extract outer membrane proteins. Shake at 35-45°C for 1-3 hours. Centrifuge at 4000-8000 rpm for 1 hour, collect the supernatant, then add ethanol to 60-80% to precipitate the outer membrane protein. After reconstitution with the injection solution, the 400KD filtrate was collected by ultrafiltration, and then concentrated by a 30KD ultrafiltration membrane. Use SDS-PAGE to detect the content of the desired type 1, 2 or 1, 3 outer membrane protein. Chromatography with Sephacryl S 200-400 to collect the required type 1, 2 or 1, 3 outer membrane proteins. Detection of protein content (Lowry method), main components and t...
Embodiment 2
[0052] Group A meningococcal polysaccharide conjugate
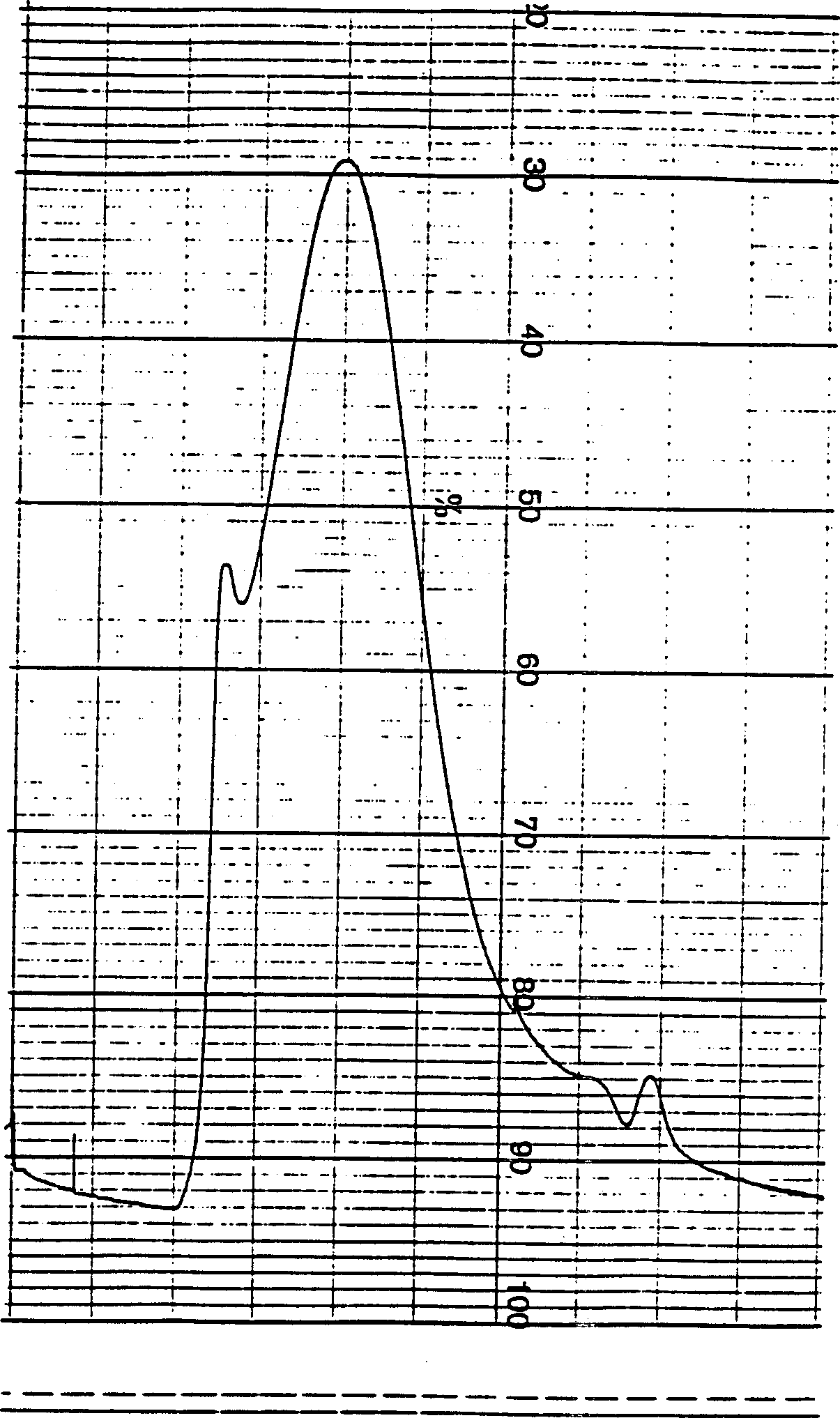
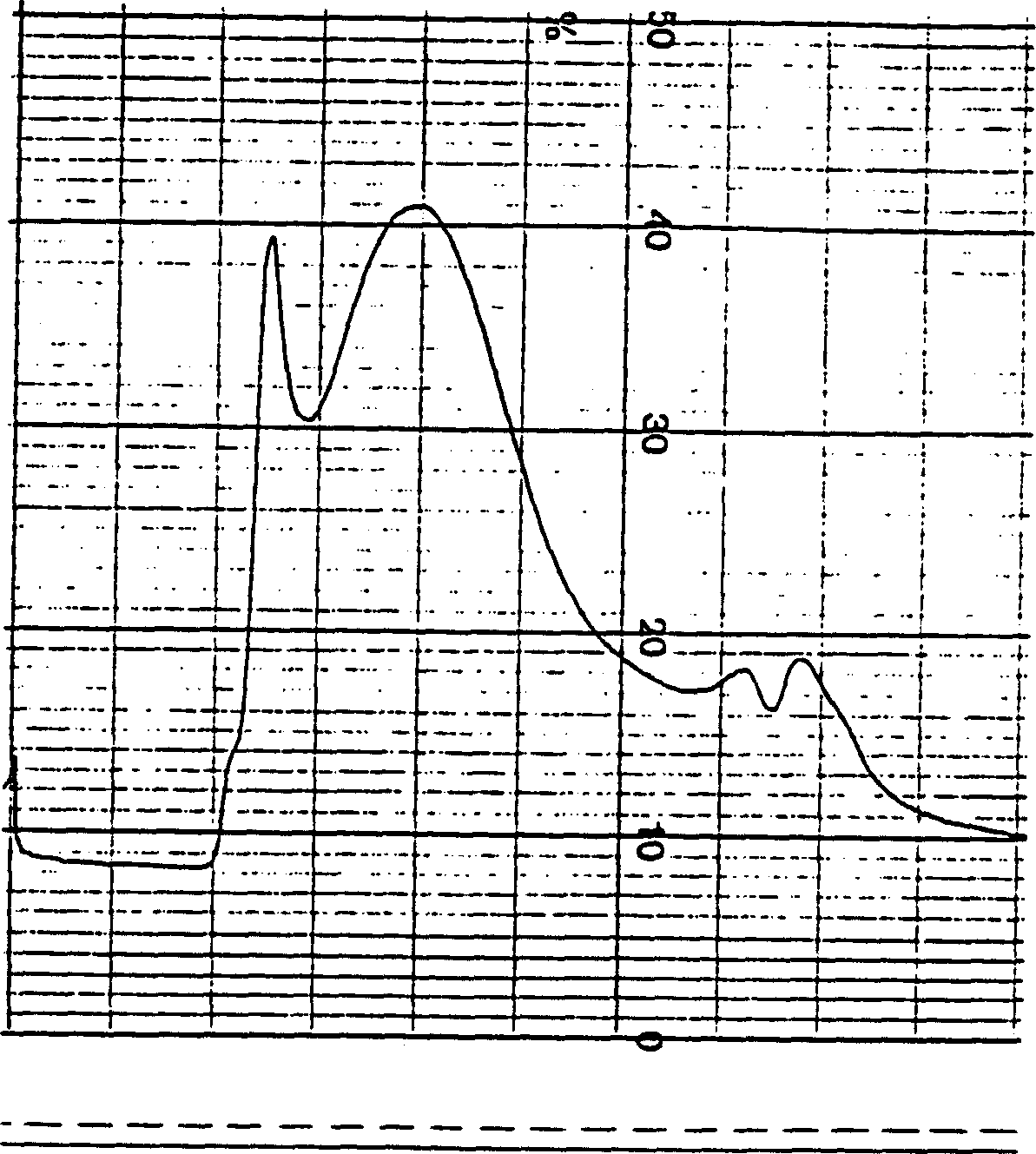
[0053] Group A meningococcus capsular polysaccharide diluted to 4mg / ml, add cyanogen bromide to activate the polysaccharide, act at 23±3℃ for 1-1.5 hours, adjust pH and maintain pH9-11, then add adiphydrazide 2.5-5mg / ml mgps, maintain pH8-10 for 10-30 minutes, stir at 2-8°C for 10-20 hours, dialyze to remove small molecular substances, take samples to measure the residual amount of cyanogen bromide and the derivatization rate of adipic hydrazide. Add an equal amount of group B meningococcal outer membrane protein and polysaccharide to mix, add carbodiimide: 15-25mg / ml mixture, act at 5-15°C for 1-1.5 hours, and adjust pH to 5-7. The coupling stock solution was dialyzed to remove small molecular substances. 300KD membrane bag ultrafiltration is concentrated, through Sepharose CL-4B column chromatography, collects the coupling compound of high molecular weight (see Figure 4 ), sterilizing filtration to detect phosphorus ...
Embodiment 3
[0055] Group C meningococcal capsular polysaccharide conjugate
[0056] Group C meningococcal polysaccharide diluted to 4 mg / ml, add cyanogen bromide (CNBr) to activate the polysaccharide, act at 20-30°C for 1-1.5 hours, adjust pH and maintain pH 9-11, then add adipic hydrazide (ADH) 2.5-5 mg / mg polysaccharide, maintain pH 8-10, react for 30 minutes, stir at 2-8°C for 10-20 hours, dialyze to remove small molecular substances. Sampling was carried out to measure residual CNBr content and ADH derivatization rate. Add an equal amount of group B meningococcal outer membrane protein and polysaccharide to mix, add carbodiimide (EDAC): 15-25mg / ml mixture, react at 5-15°C for 1-1.5 hours, adjust pH to 5-7. The coupling stock solution was dialyzed to remove small molecular substances, concentrated by ultrafiltration in a 300KD membrane bag, and subjected to Sepharose CL-4B column chromatography to collect high molecular weight coupling compounds (see Figure 5 ), sterilizing filtrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com