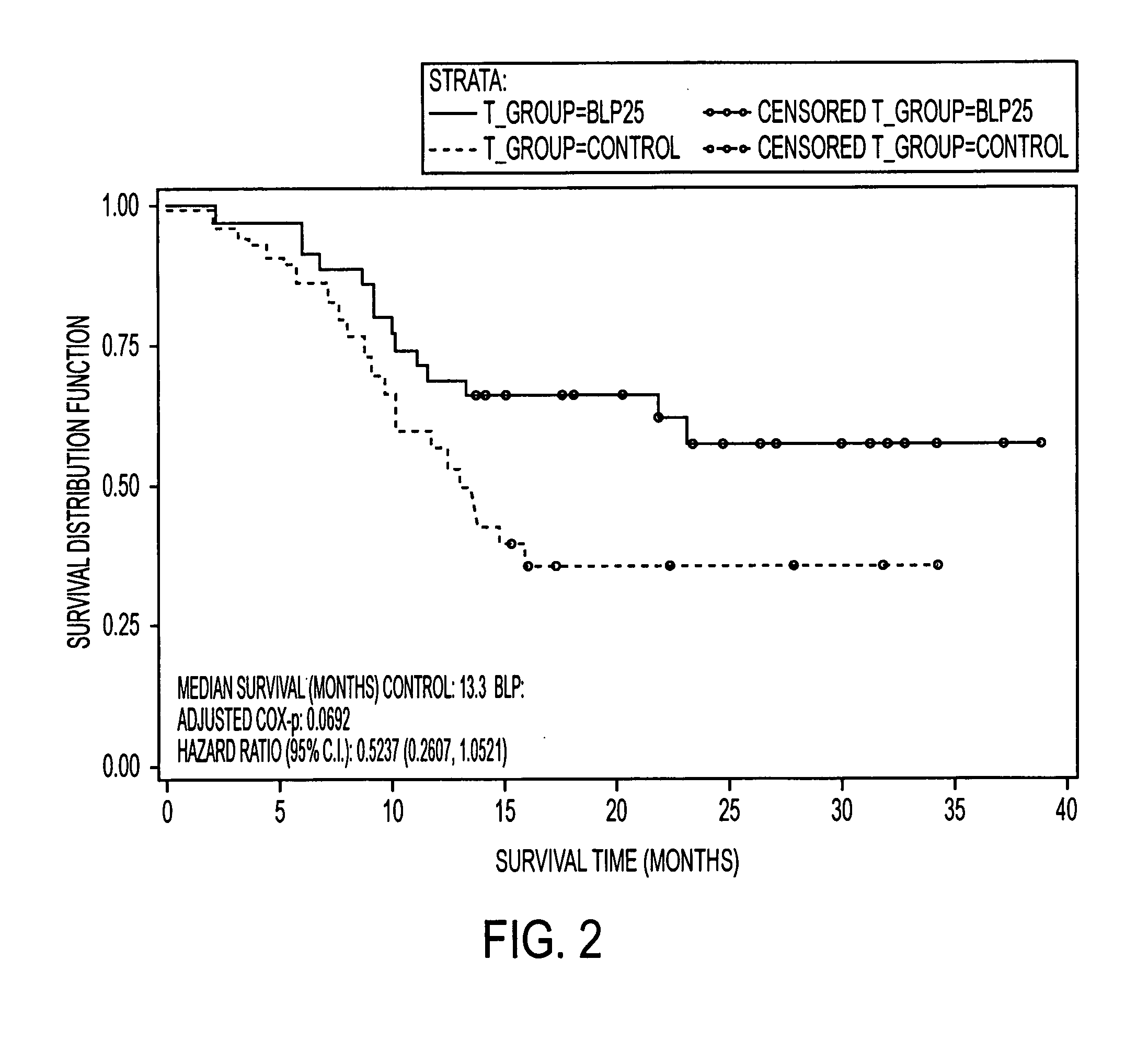
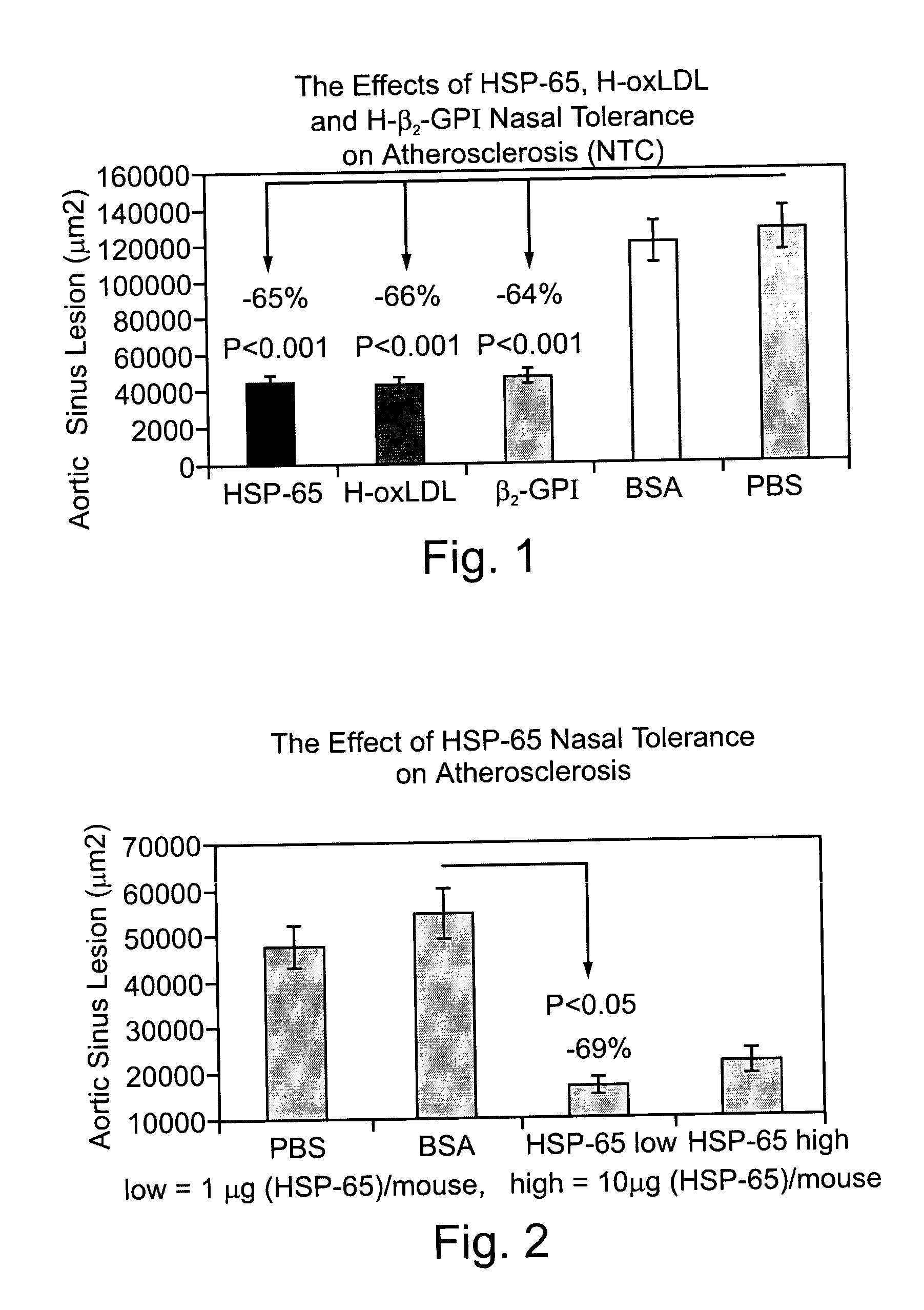
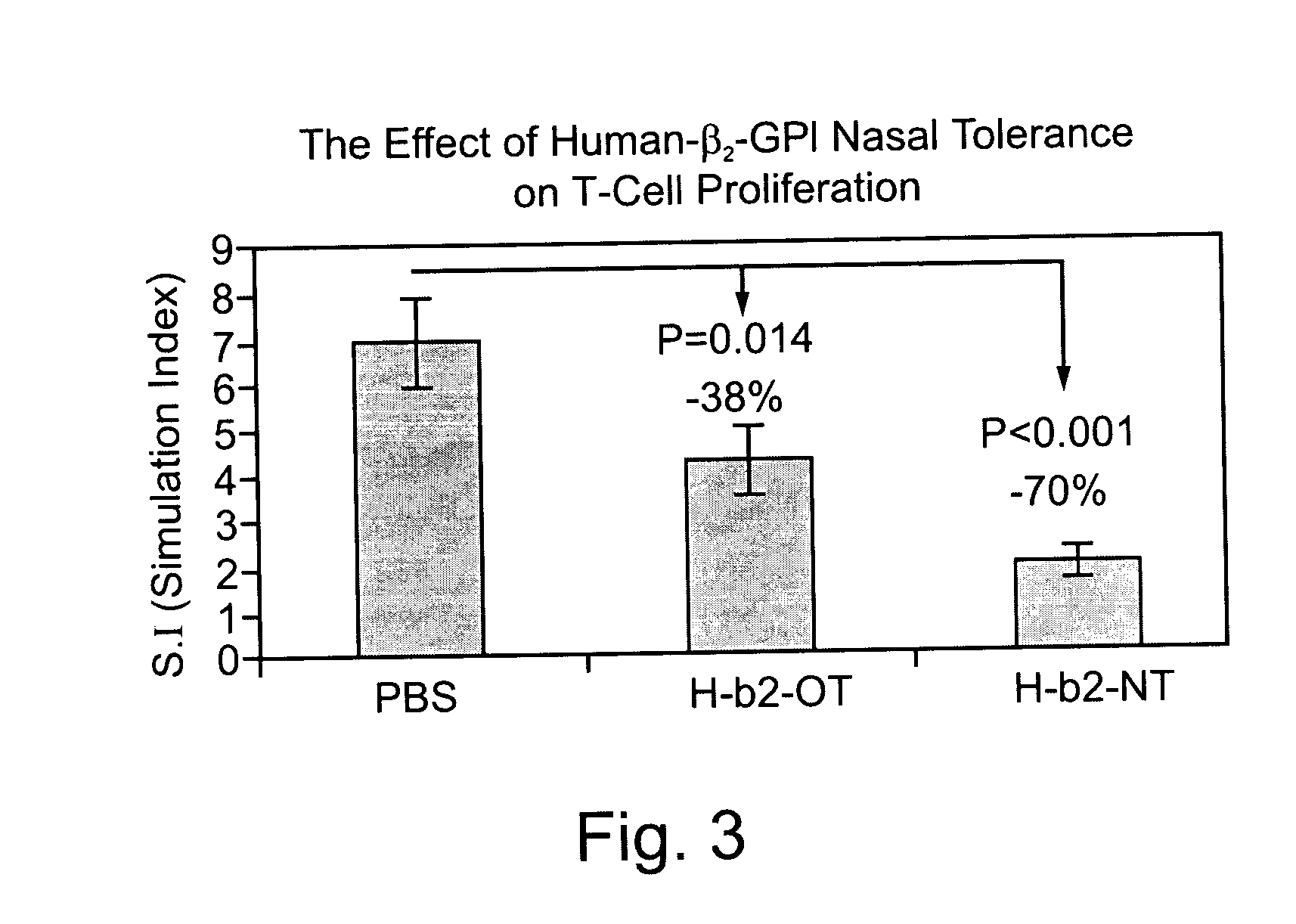
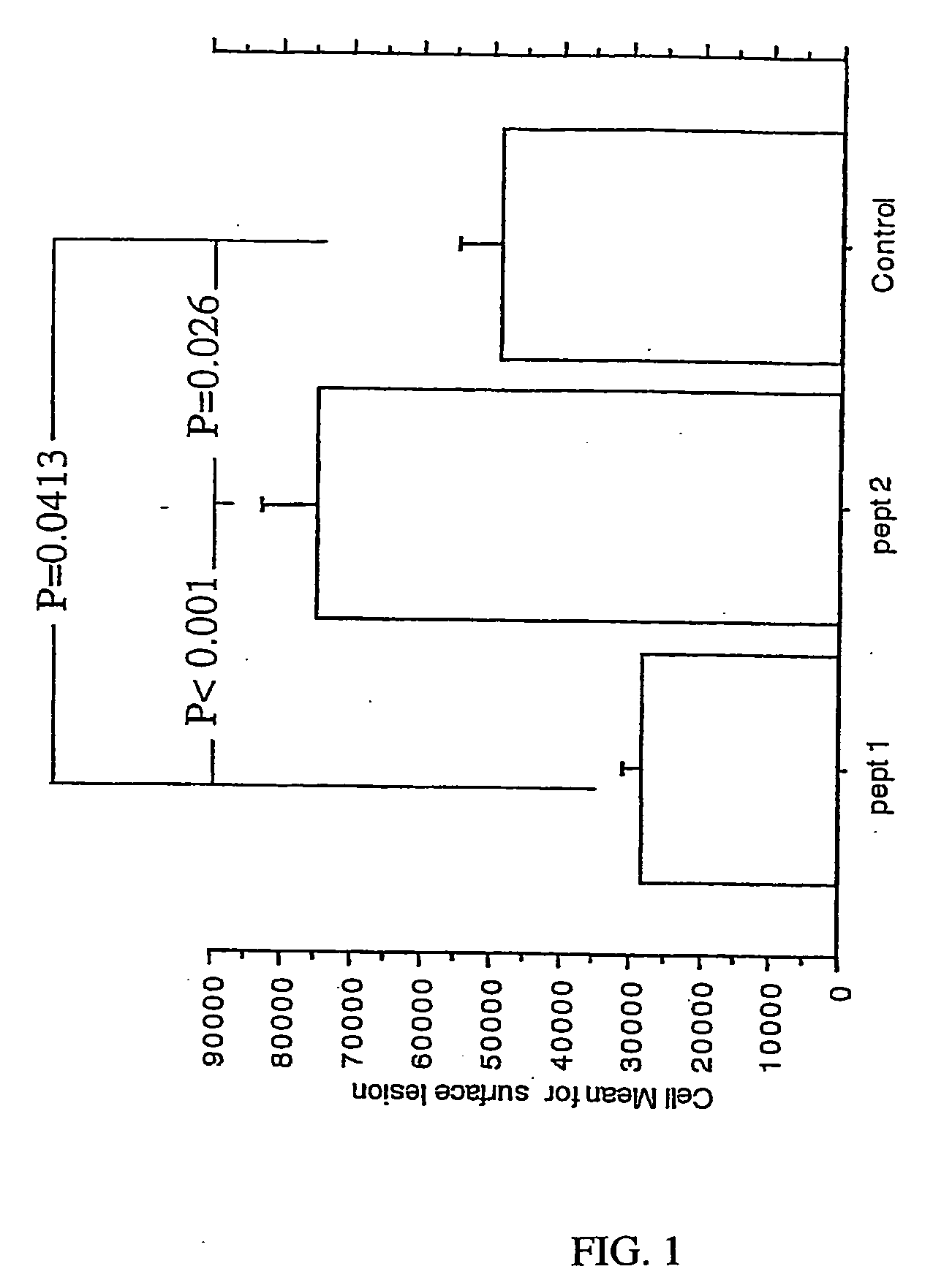
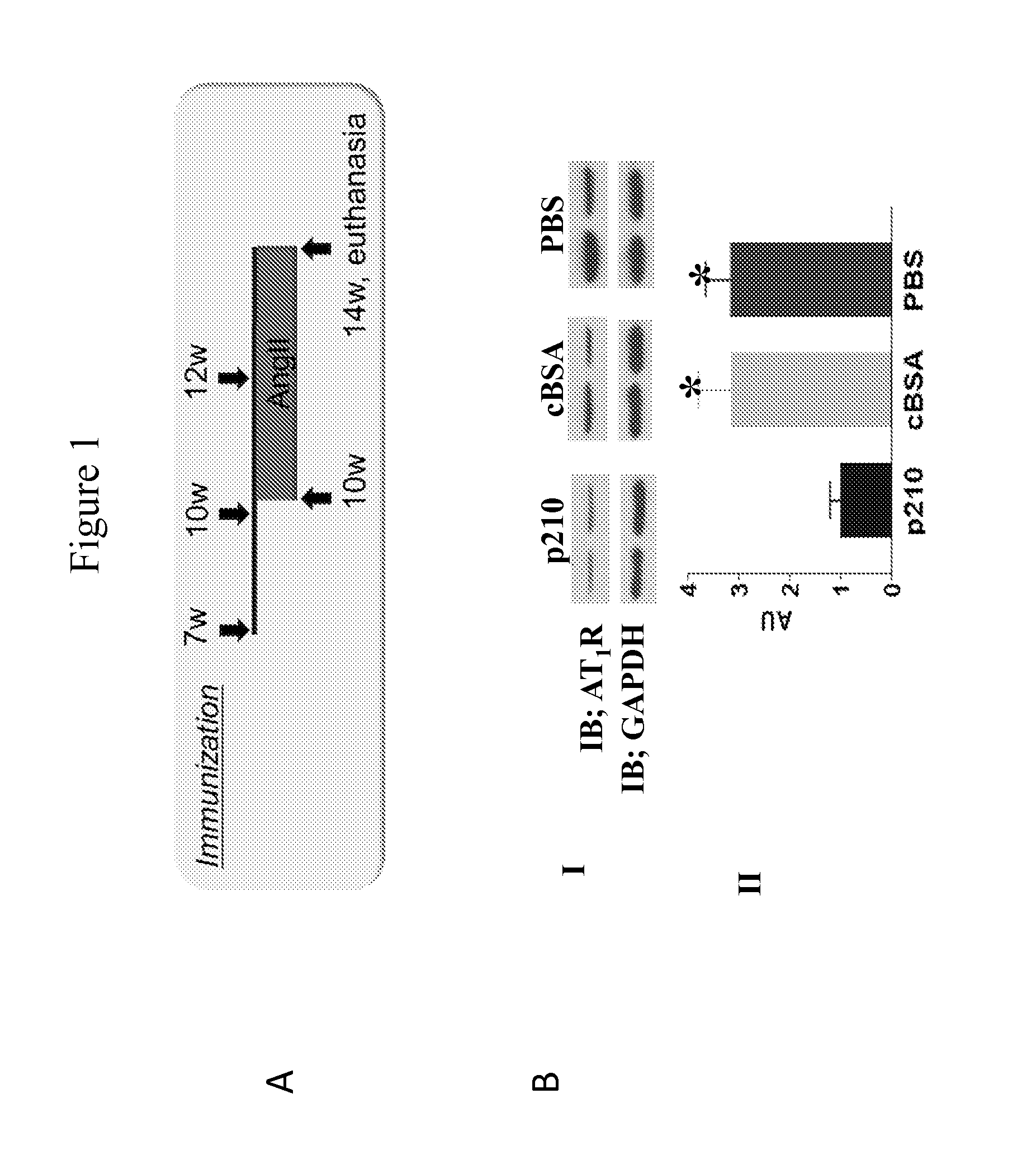
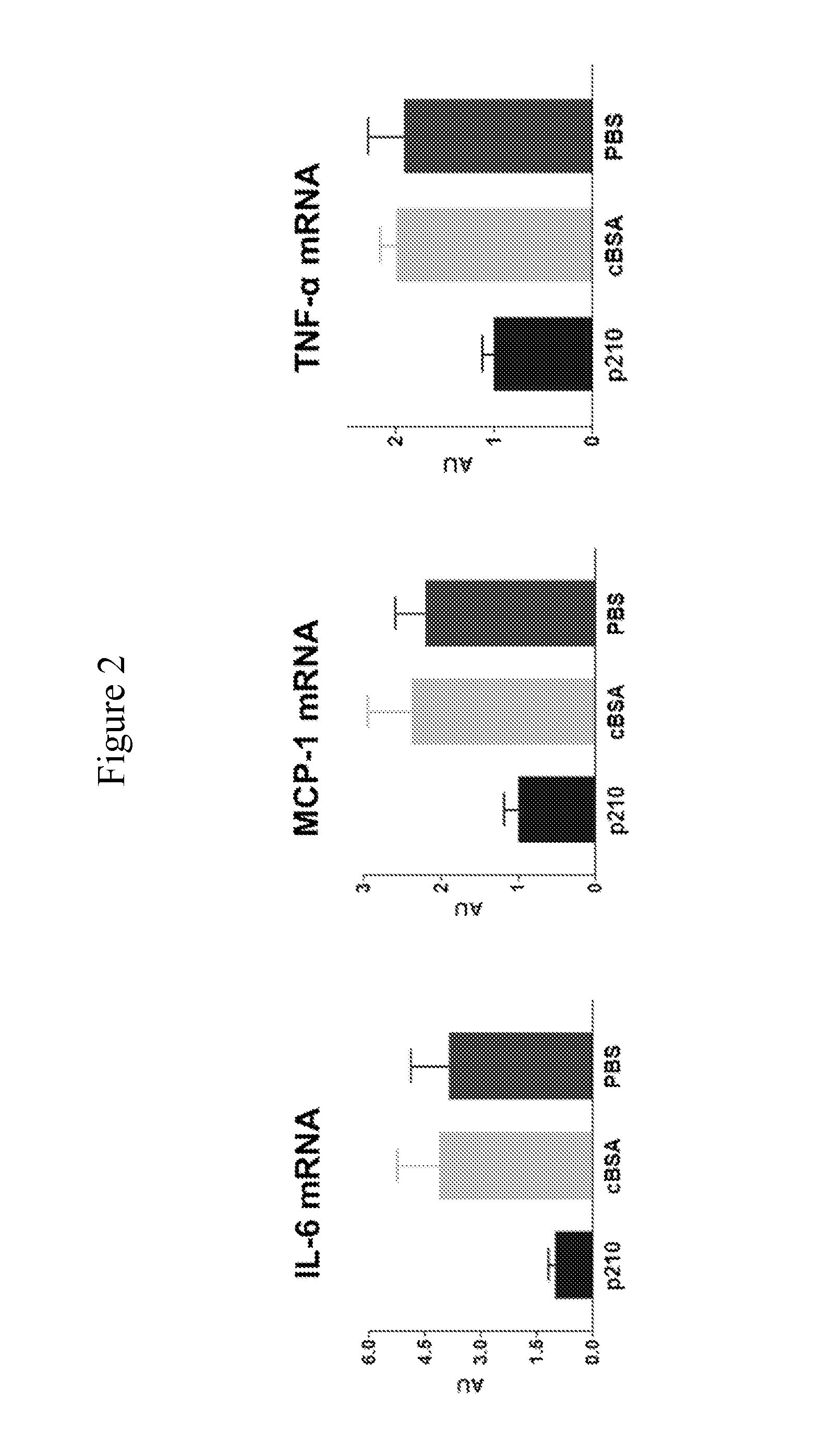
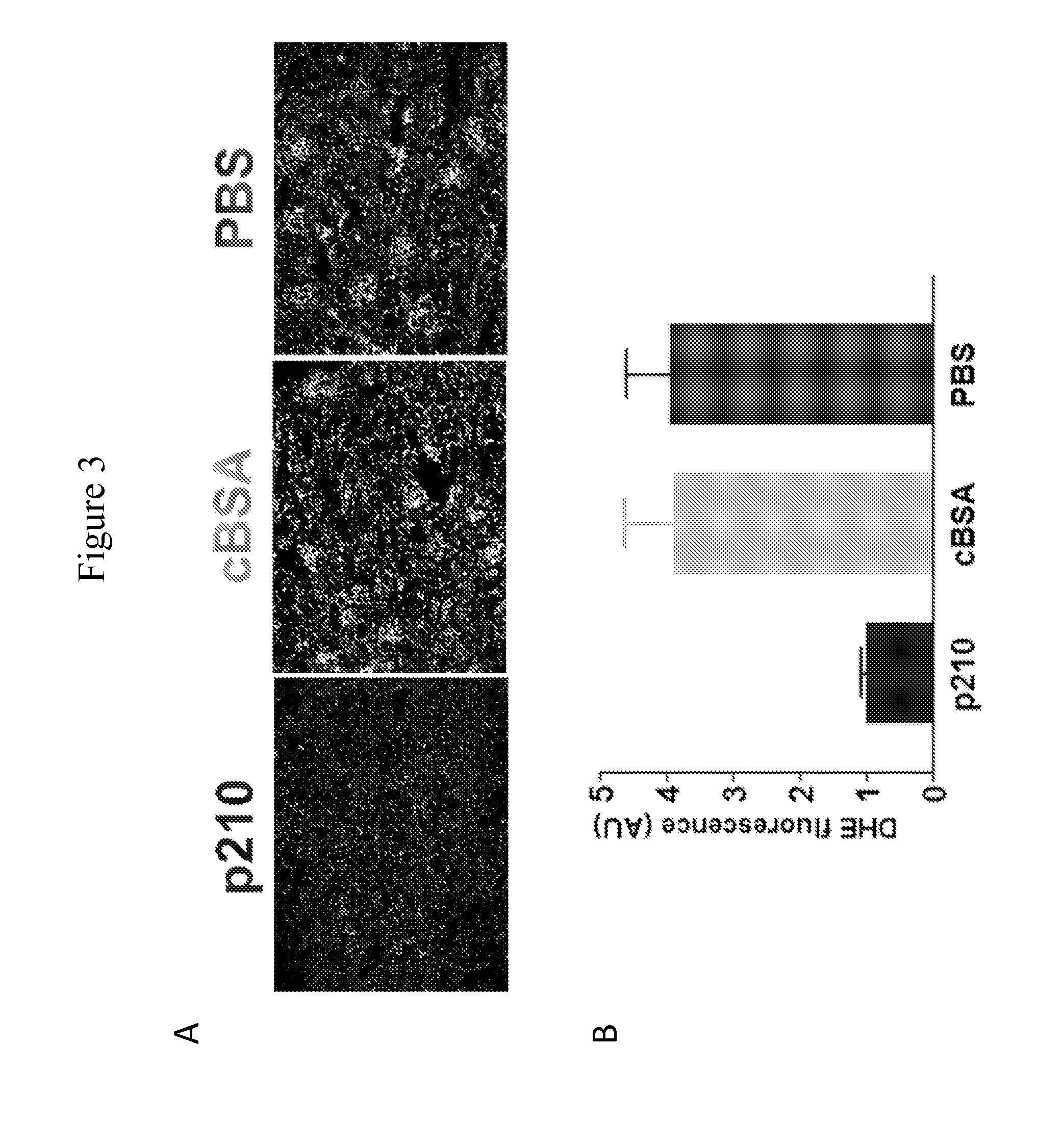
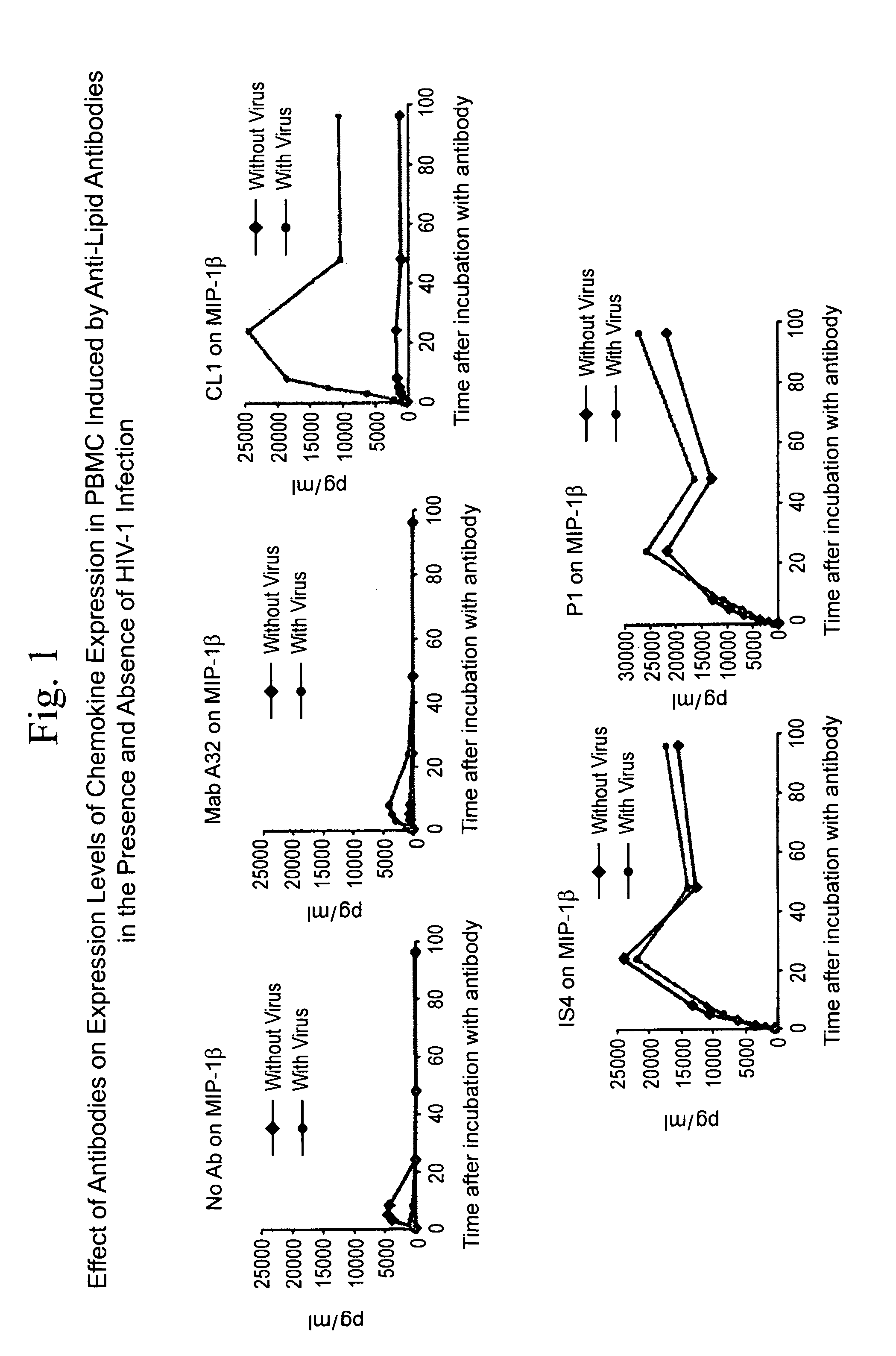
Patents
Literature
43results about "Lipid/lipoprotein ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Conjugate vaccines for non-proteinaceous antigens
InactiveUS20070231344A1Strong and rapid effectStrong and rapid immune responseBacterial antigen ingredientsLipid/lipoprotein ingredientsAntigenConjugate vaccine
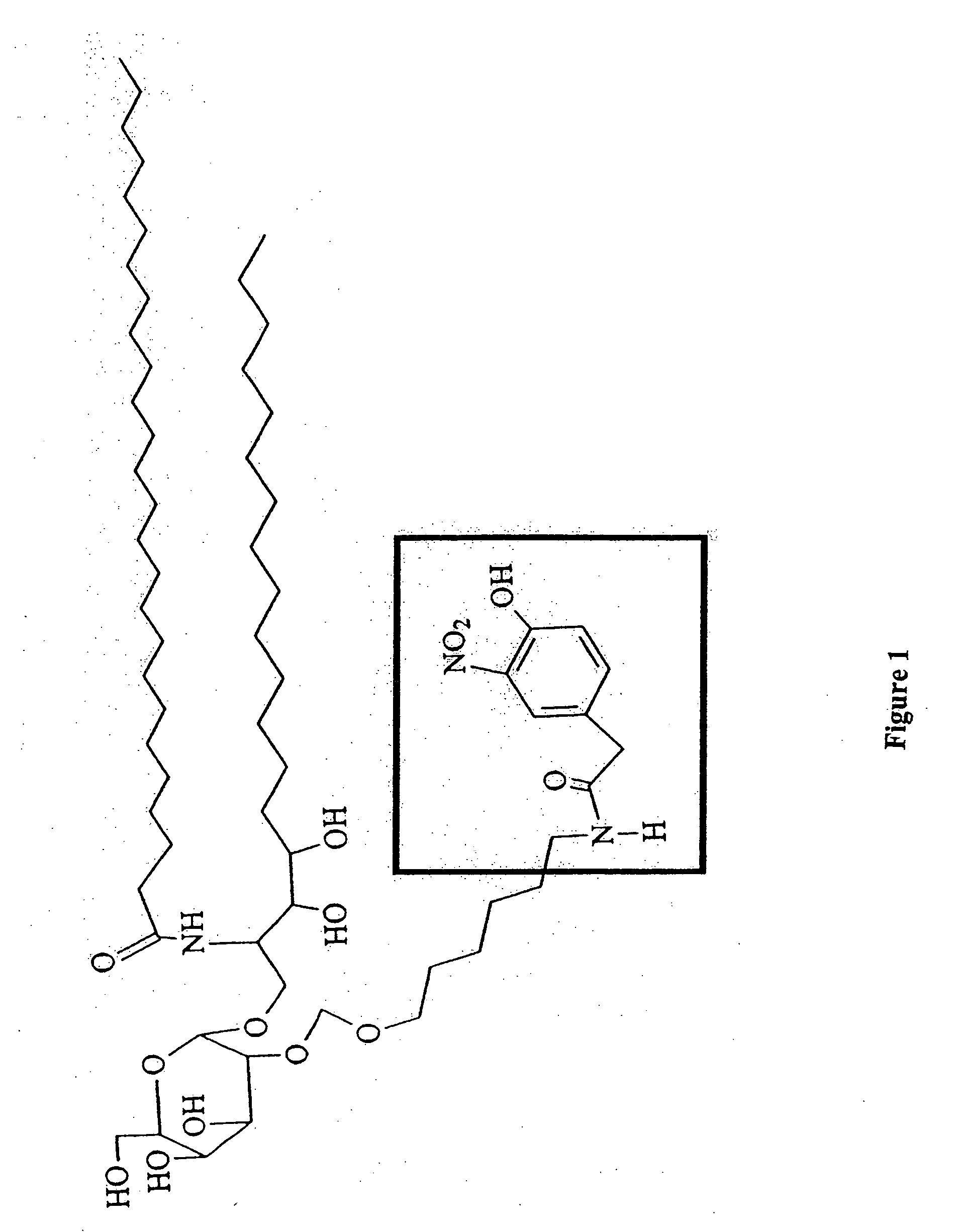
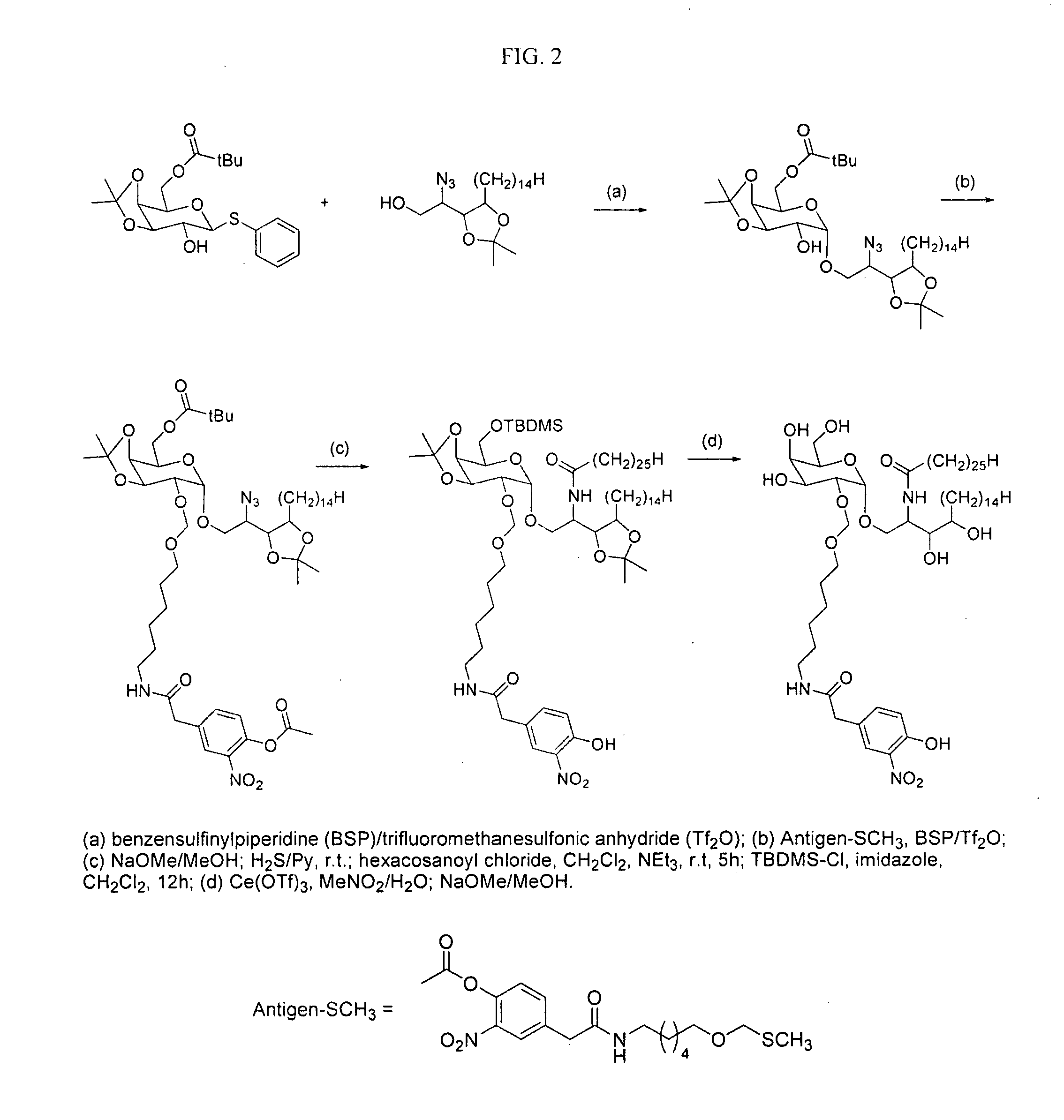
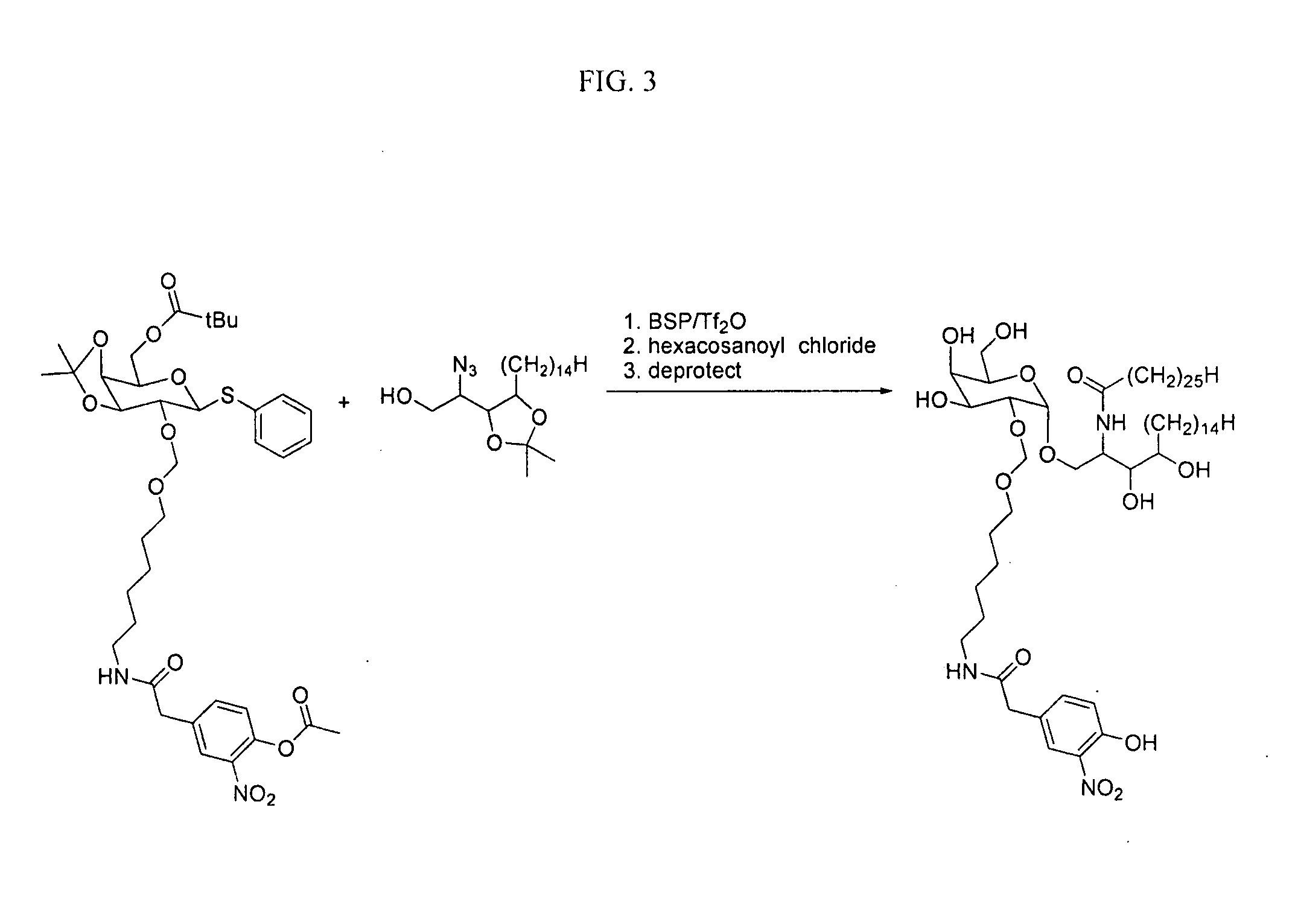
The present invention is directed to pharmaceutical compositions that can be used to immunize subjects using, for example, lipid, glycan, or nucleic acid antigens. These antigens are conjugated to a glycosphingolipid.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Atherosclerosis vaccine
InactiveUS20040002111A1Peptide preparation methodsLipid/lipoprotein ingredientsAntigenComplementarity determining region
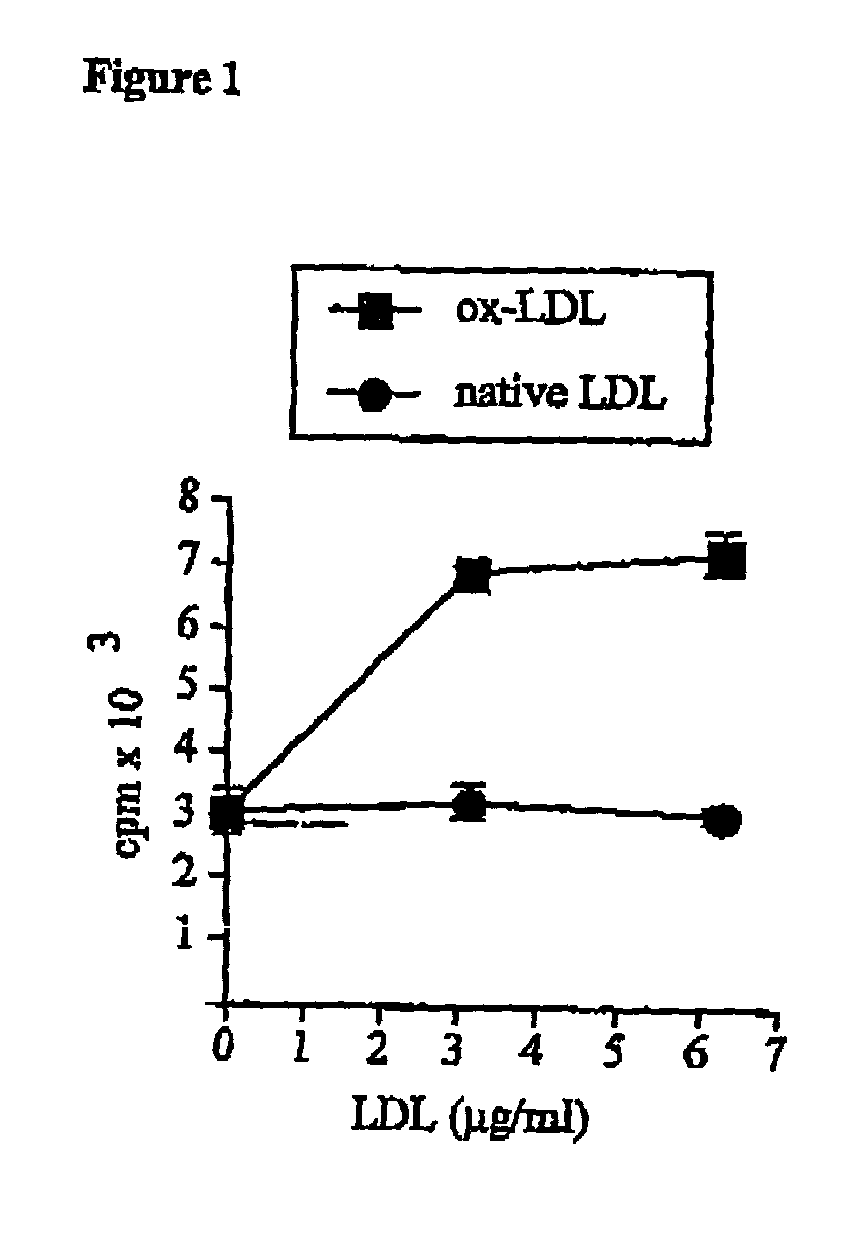
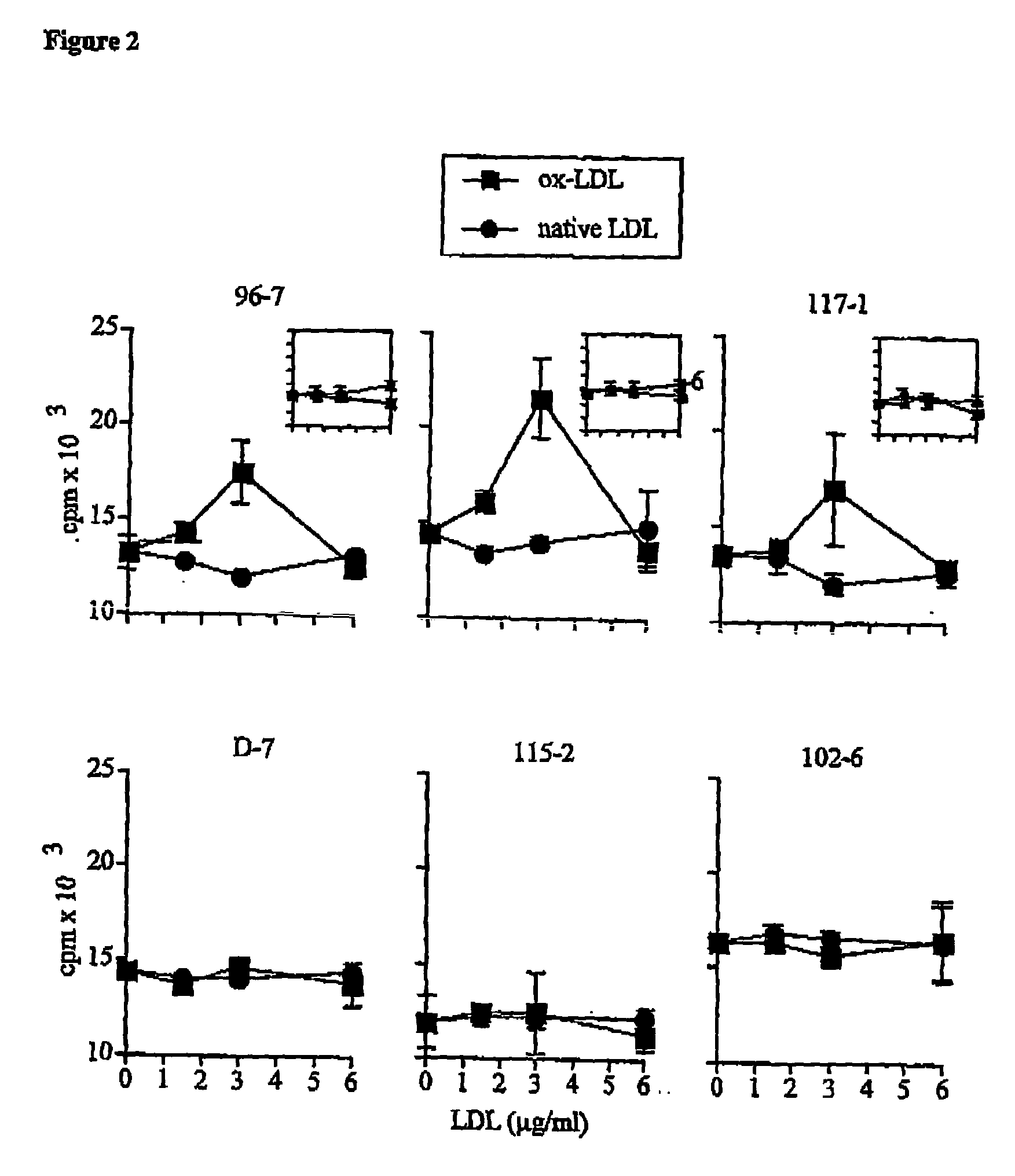
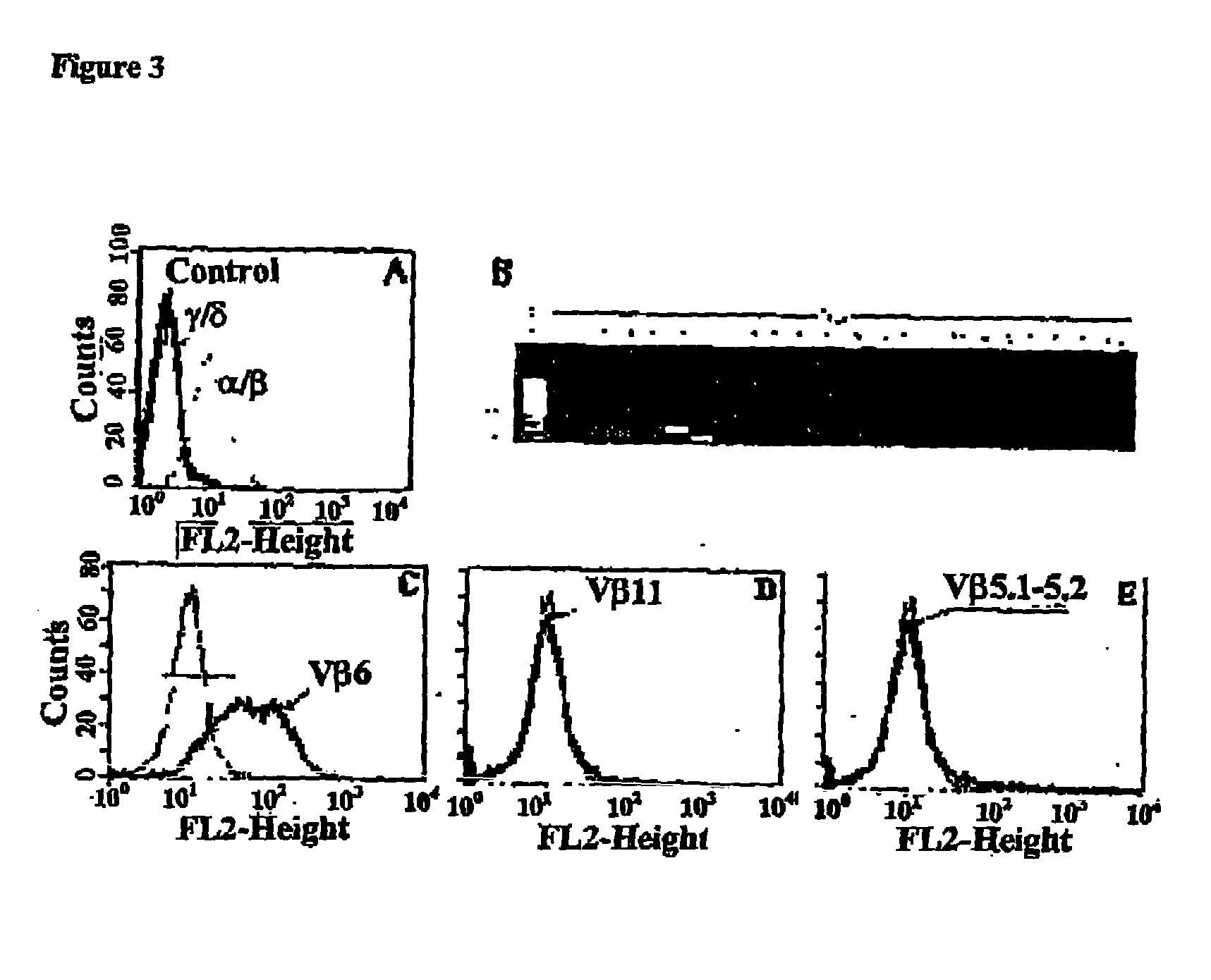
The present invention relates to an antigenic composition capable of eliciting antibodies by interacting with alphabeta chains of a T cell receptor (TcR), which composition is comprised of a peptide-aldehyde conjugate. The aldehyde portion may be a dialdehyde, such as malondialdehyde (MDA), or a monoaldehyde, such as 4-hydroxynonenal (4-HNE), while the peptide portion preferably comprises at least one lysine residue. The antigenic composition according to the invention is capable of recognizing and interacting with a TcR having a complementarity-determining region 3 (CDR3) of alpha10 and beta6 chains that comprises a cluster of charged and polar amino acids. The invention also relates to a method of producing a vaccine against atherosclerosis by screening of a library of candidate compounds for their ability to bind to a conjugate of oxidized LDL and a dialdehyde as well as to such a vaccine as such.
Owner:CARDIOVAX
Exosome ligands, their preparation and uses
InactiveUS20090148460A1Improved exosome blocking agentHigh affinityAntibacterial agentsBiocideAntigenExosome
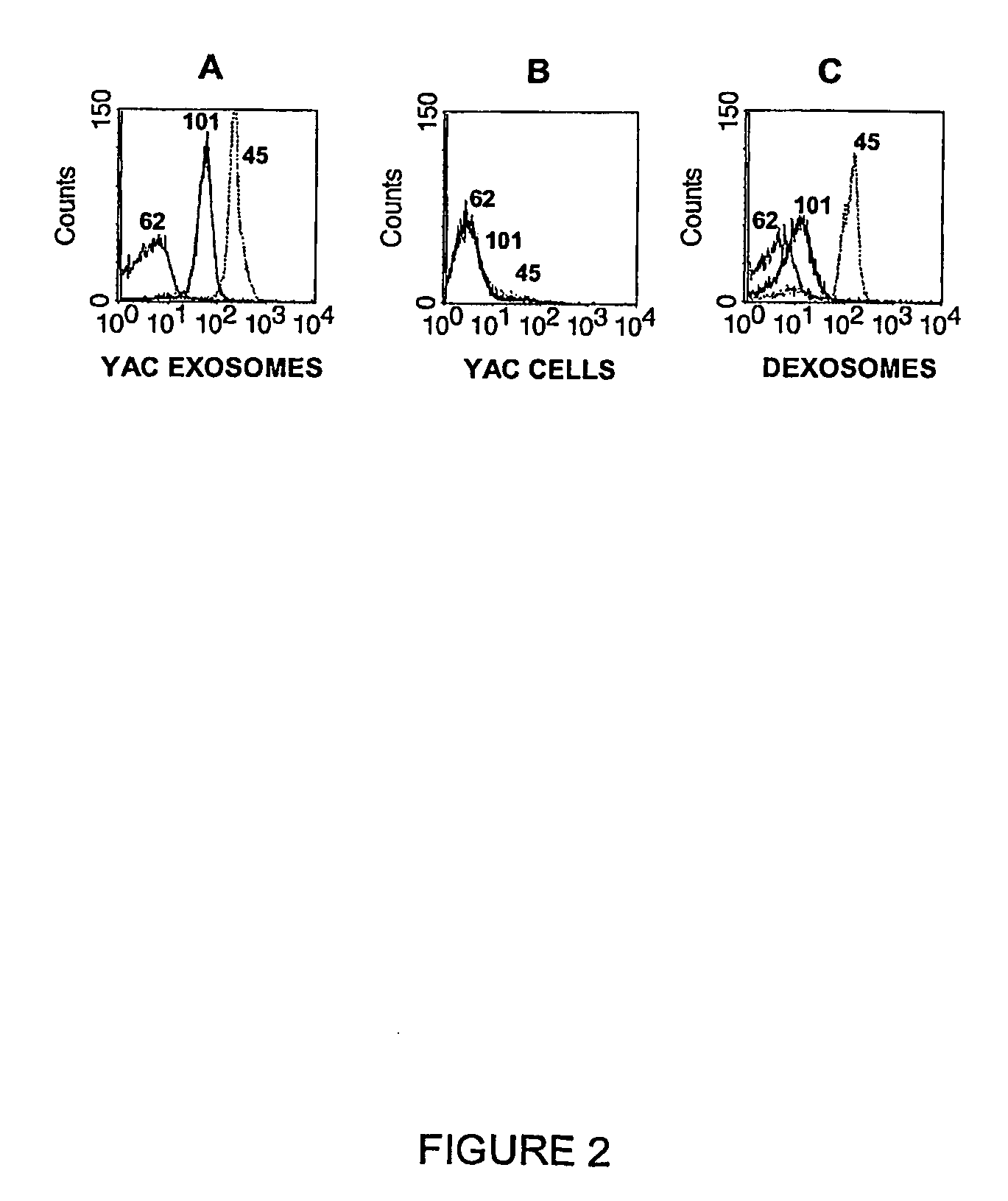
The present invention relates to exosome-specific ligands and compositions comprising the same. The invention also relates to methods of generating said ligands and compositions, to methods of using said ligands or compositions, e.g., to block the exosome pathway or to detect and / or characterize exosomes in a sample or subject, as well as to the antigens contacted by said ligands or compositions. The application can be used in experimental, research, therapeutic, prophylactic or diagnostic areas.
Owner:EXOTHERA
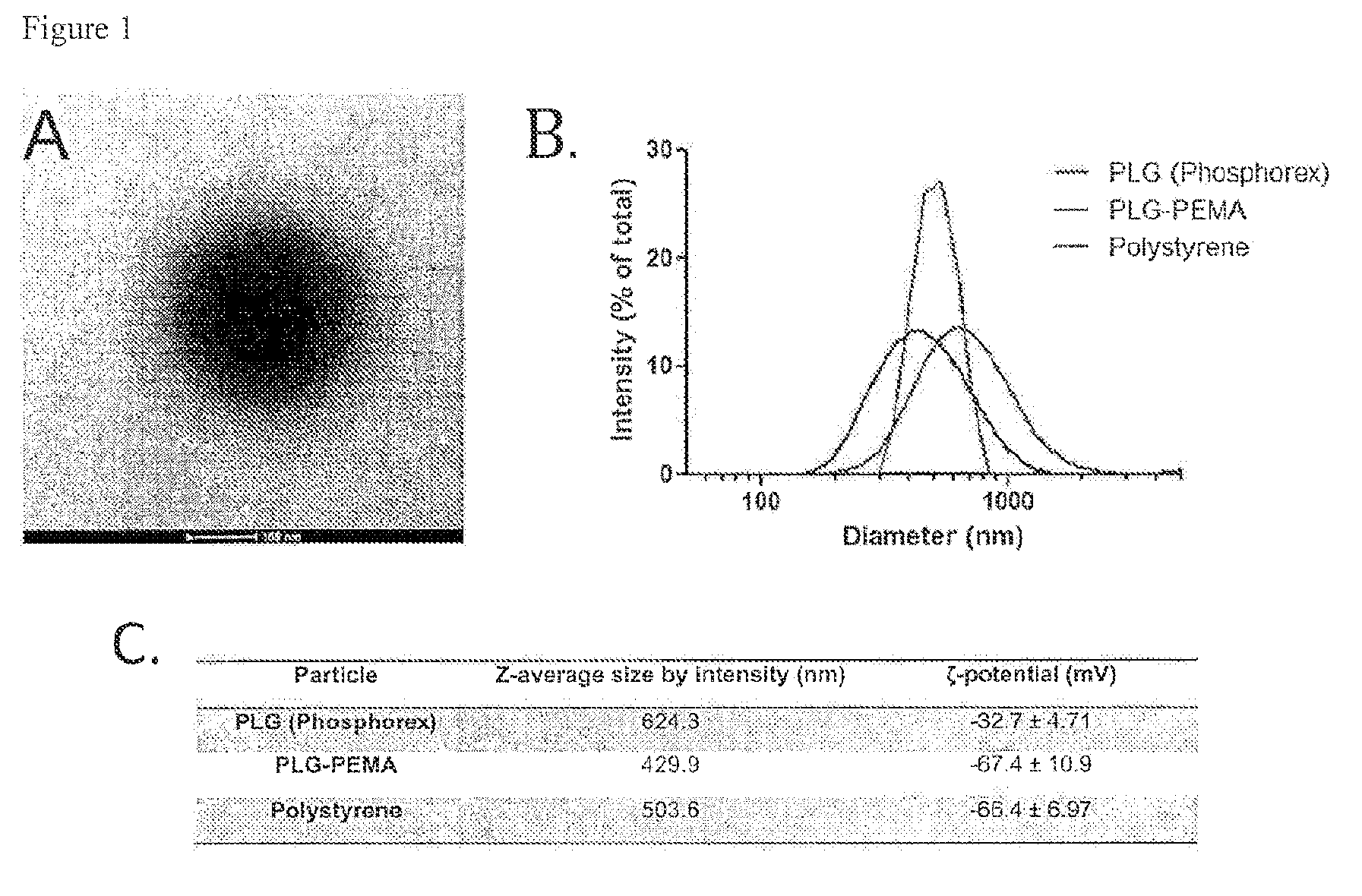
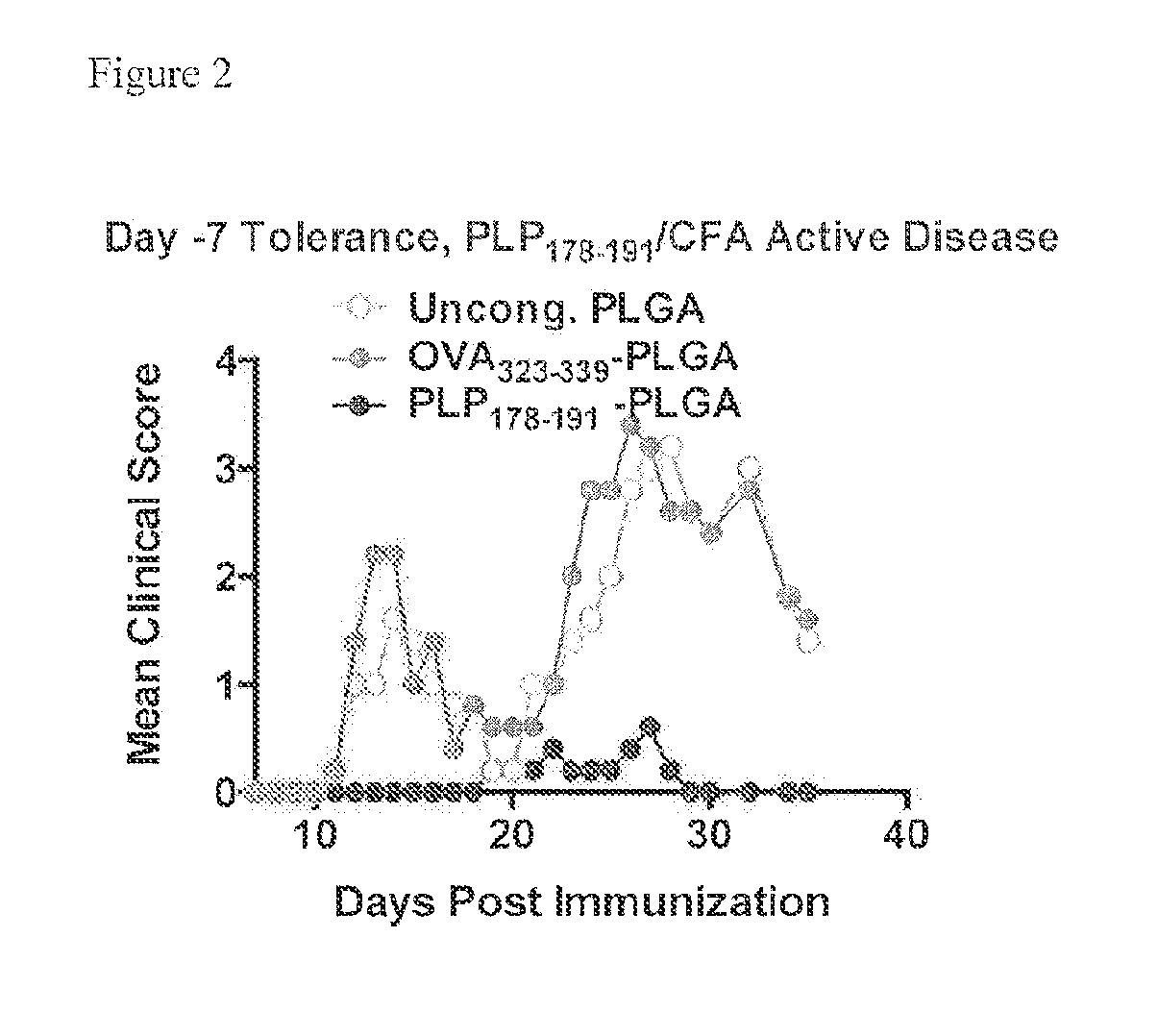
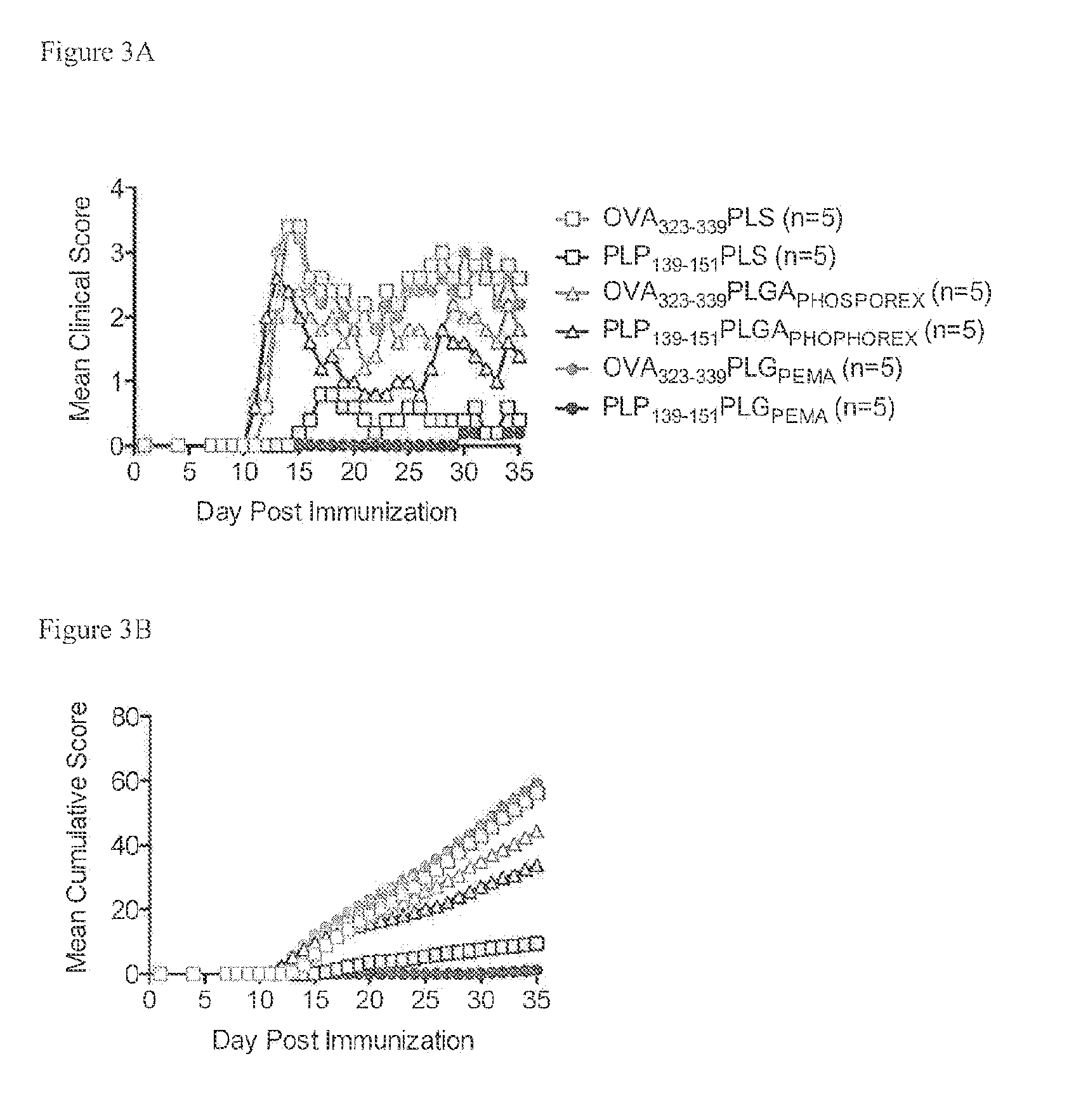
Peptide conjugated particles
The present invention provides compositions comprising peptide-coupled biodegradable poly(lactide-co-glycolide) (PLG) particles In particular, PLG particles are surface-functionalized to allow for coupling of peptide molecules to the surface of the particles (e.g., for use in eliciting induction of immunological tolerance).
Owner:NORTHWESTERN UNIV
Peptide-based immunization therapy for treatment of atherosclerosis
InactiveUS20080070265A1Reduce developmentStrong immune responseApolipeptidesMicrobiological testing/measurementMammalTherapeutic treatment
The present invention relates to a fragment of apolipoprotein B, for immunization for prophylactic or therapeutic treatment of mammals, including humans, against ischemic cardiovascular diseases, in particular myocardial infarction or stroke, as well as diagnosing the presence or absence of antibodies related to increased or decreased risk of developing ischemic cardiovascular diseases including stroke, using said peptide in an assay, pharmaceutical compositions comprising the peptide. The invention further encompasses a particular peptide sequence aggravating disease, which sequence then can be used for diagnostic assays.
Owner:CARDIOVAX
Method of treating patients with a mucinous glycoprotein (MUC-1) vaccine
InactiveUS20070014844A1Keep for a long timeBiocideOrganic active ingredientsProstate cancerGlycoprotein
Owner:ONCOTHYREON
Methods employing and compositions containing plaque associated molecules for prevention and treatment of atherosclerosis
InactiveUS20040047870A1Improve toleranceInhibiting atheroslerosis and other plaque related diseasesBiocideOrganic active ingredientsDiseaseInflammation Process
Methods and compositions employing plaque associated molecules effective in inducing mucosal tolerance and inhibiting inflammatory processes contributing to atheromatous vascular disease and sequalae are provided.
Owner:VASCULAR BIOGENICS
Peptide-based immunization therapy for treatment of atherosclerosis
InactiveUS20060233817A1Strong immune responseGood effectPeptide/protein ingredientsDisease diagnosisMammalTherapeutic treatment
The present invention relates to a fragment of apolipoprotein B, for immunization for prophylactic or therapeutic treatment of mammals, including humans, against ischemic cardiovascular diseases, in particular myocardial infarction or stroke, as well as diagnosing the presence or absence of antibodies related to increased or decreased risk of developing ischemic cardiovascular diseases including stroke, using said peptide in an assay, pharmaceutical compositions comprising the peptide. The invention further encompasses a particular peptide sequence aggravating disease, which sequence then can be used for diagnostic assays.
Owner:CARDIOVAX
Compositions and methods for treatment of kidney diseases
ActiveUS20140308306A1Induce and enhance immune responseEnhance immune responseApolipeptidesPeptide/protein ingredientsNephrosisVaccination
The invention provides compositions comprising immunogenic fragments of ApoB-100 for eliciting an immune response in a subject or vaccinating a subject, so as to treat, prevent, 5 inhibit and / or reduce symptoms of kidney diseases in the subject. The compositions include immunogenic fragments of ApoB-100, CD8+ T cells activated with immunogenic fragments of ApoB-100 or a combination thereof.
Owner:CARDIOVAX
Synthetic, self adjuvanting vaccines
The present invention relates generally to the field of immunotherapy, and more particularly to immunomedicaments in the form of lipopeptides which induce an antibody response to drugs of dependence, and uses thereof in the treatment and prevention of drug addiction.
Owner:BAYLOR COLLEGE OF MEDICINE +1
Methods employing and compositions containing plaque associated molecules for prevention and treatment of atherosclerosis
InactiveUS7279459B2Reduce reactivityImprove toleranceOrganic active ingredientsBiocideVascular diseaseAtheroma
Methods and compositions employing plaque associated molecules effective in inducing mucosal tolerance and inhibiting inflammatory processes contributing to atheromatous vascular disease and sequalae are provided.
Owner:VASCULAR BIOGENICS
Methods to alter the tumor microenvironment for effective cancer immunotherapy
InactiveUS20180221475A1Growing populationEasy to demonstrateViral antigen ingredientsAntibody ingredientsRegulatory T cellTumor microenvironment
Methods and compositions for altering the microenvironment of a tumor are provided. The methods comprise reducing the population of tumor-residing immune suppressive regulatory T-cells, increasing the population of tumor lysing T-cells (such as CD8+ T-cells) and improving the efficacy of cancer immunotherapy. The compositions comprise the use of cationic lipids optionally combined with autologous antigens, non-autologous antigens, or tumor-associated antigens.
Owner:PDS BIOTECH
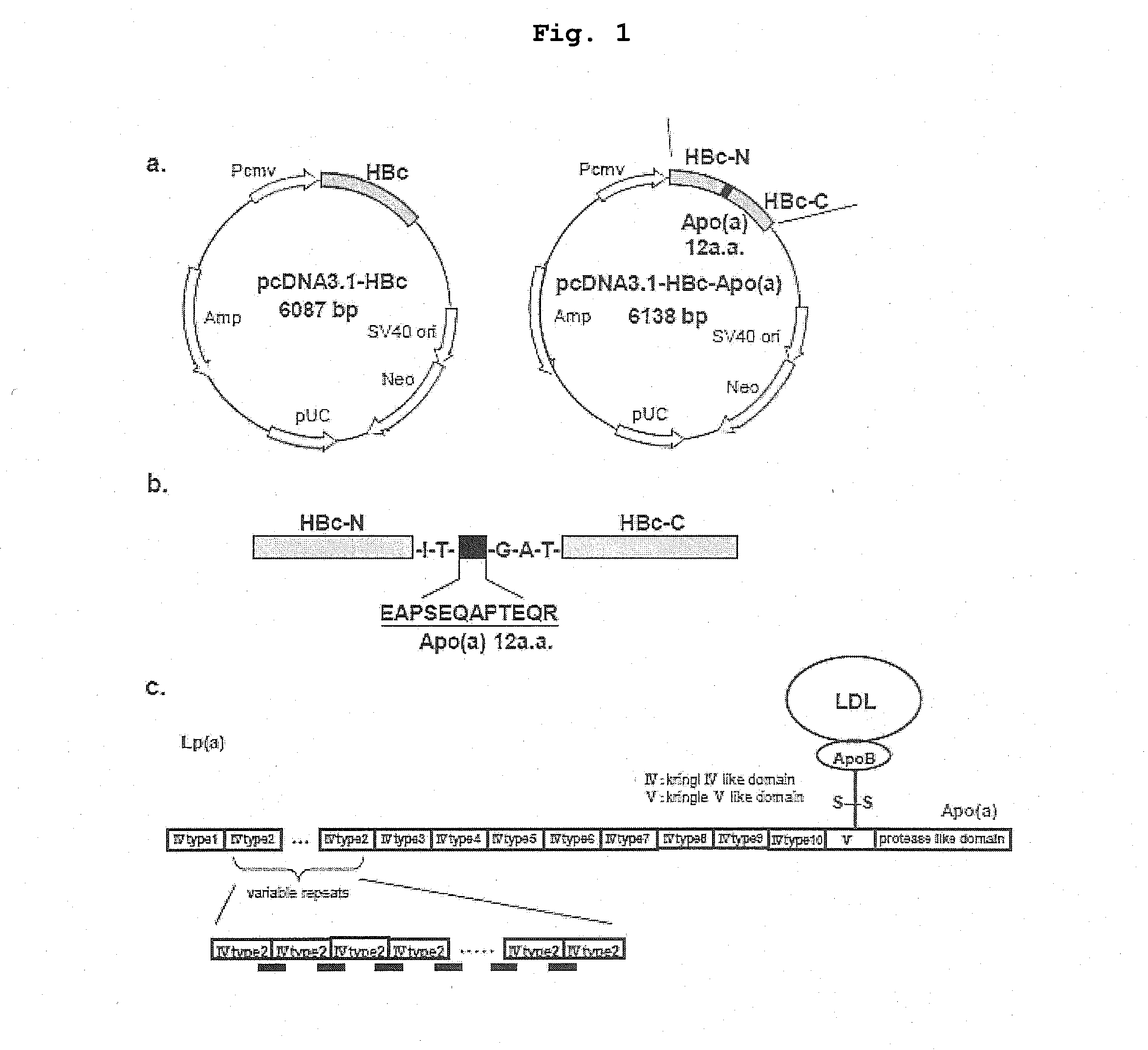
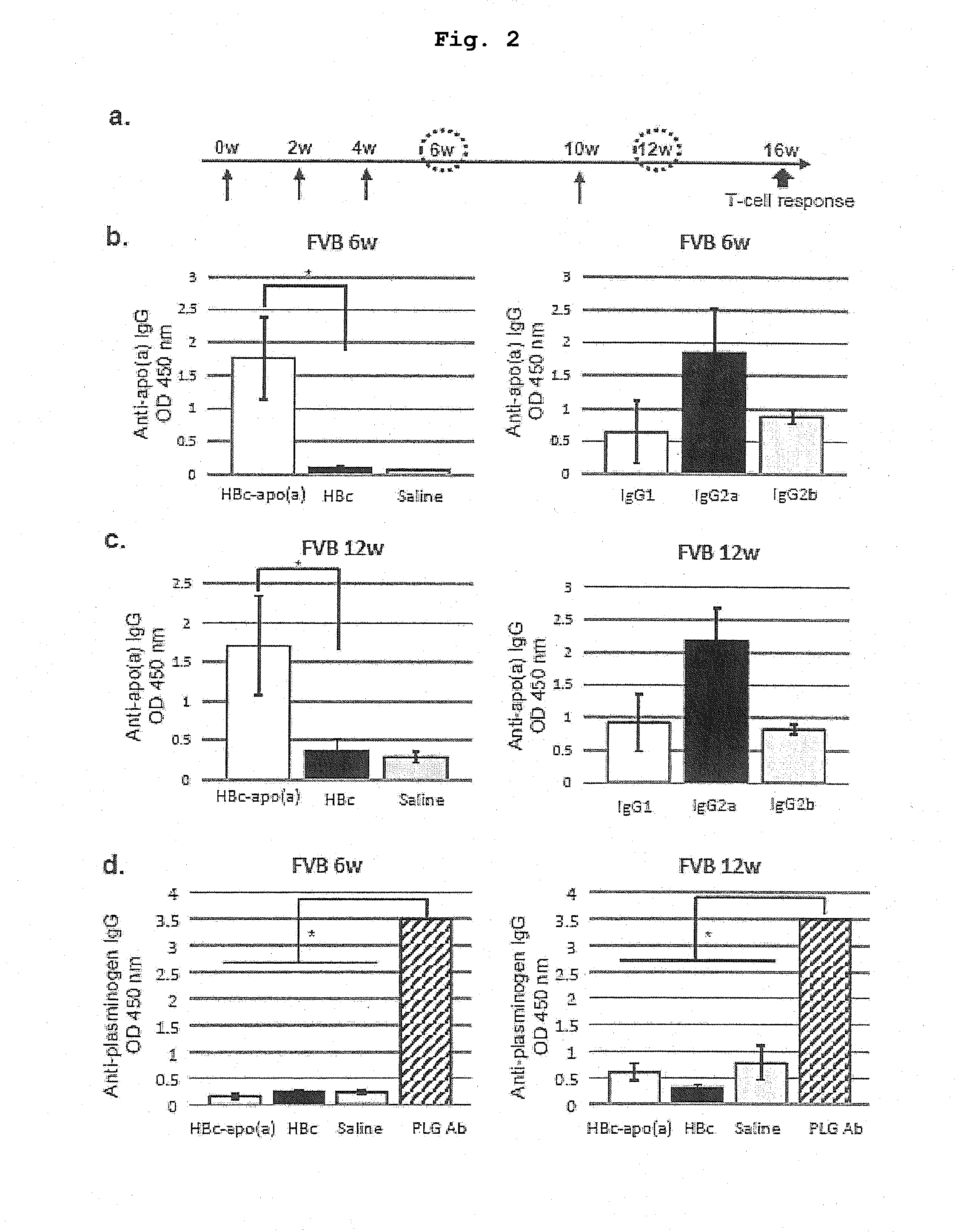
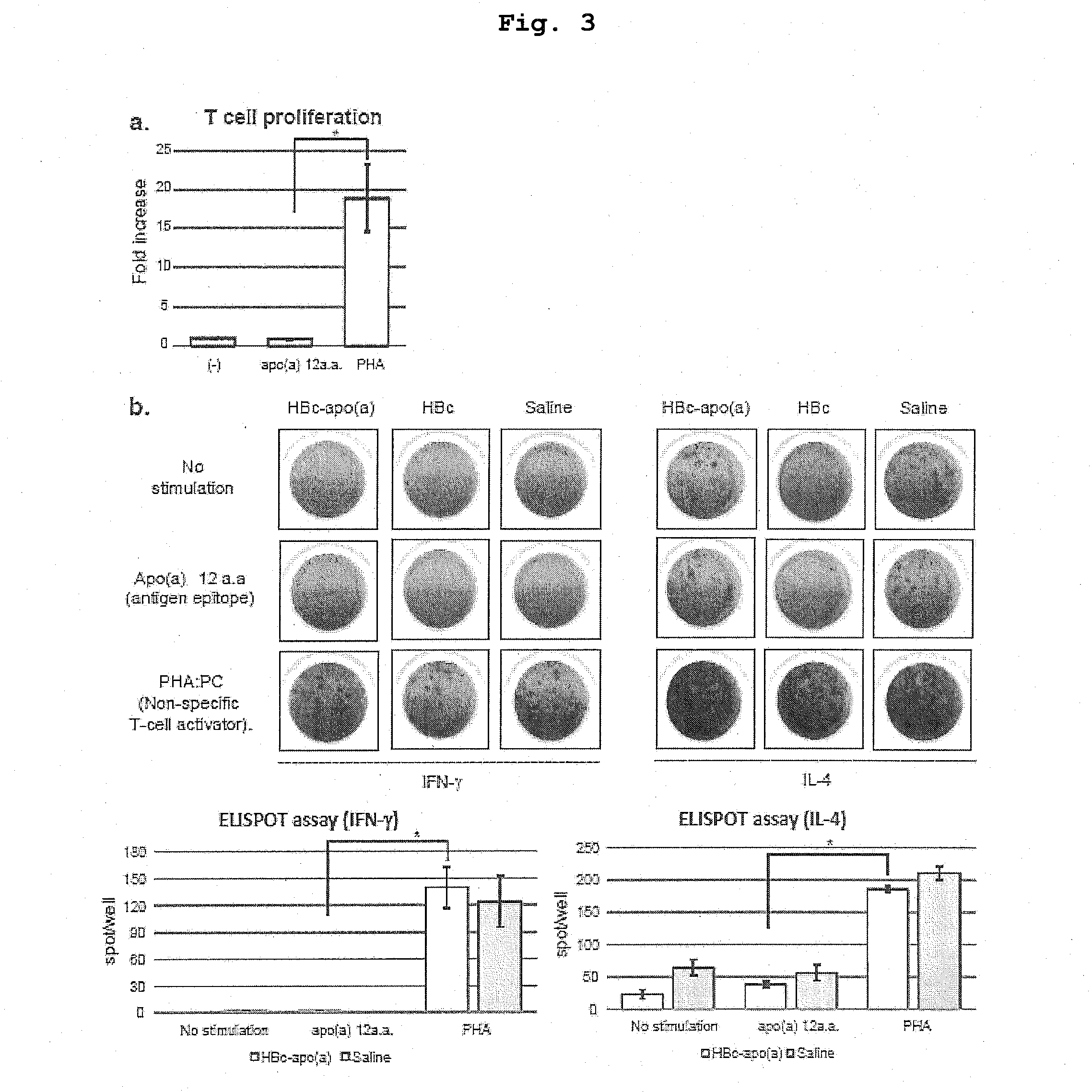
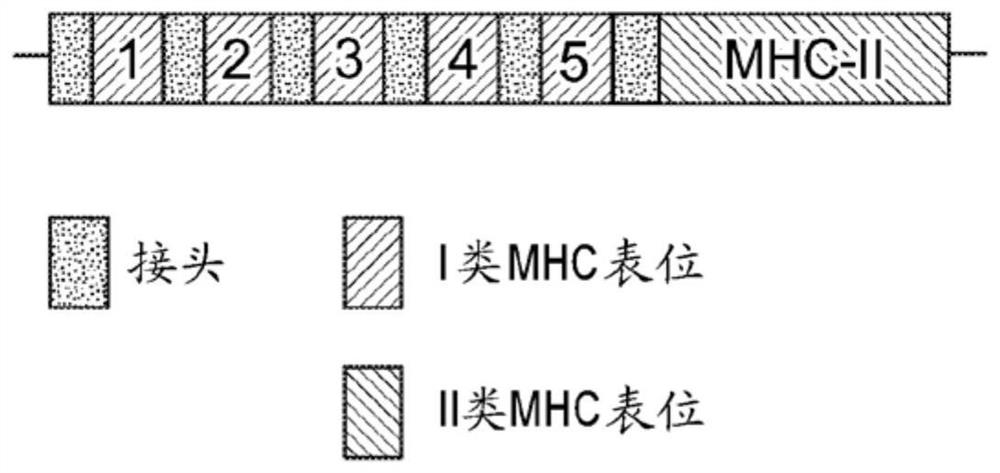
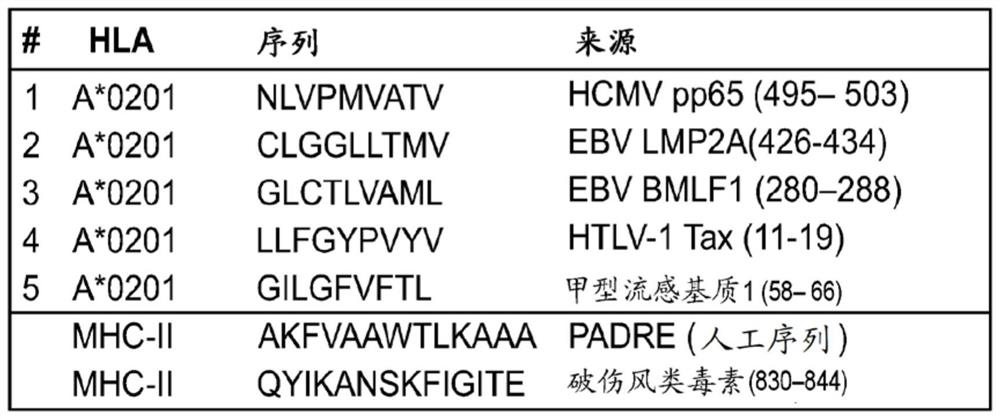
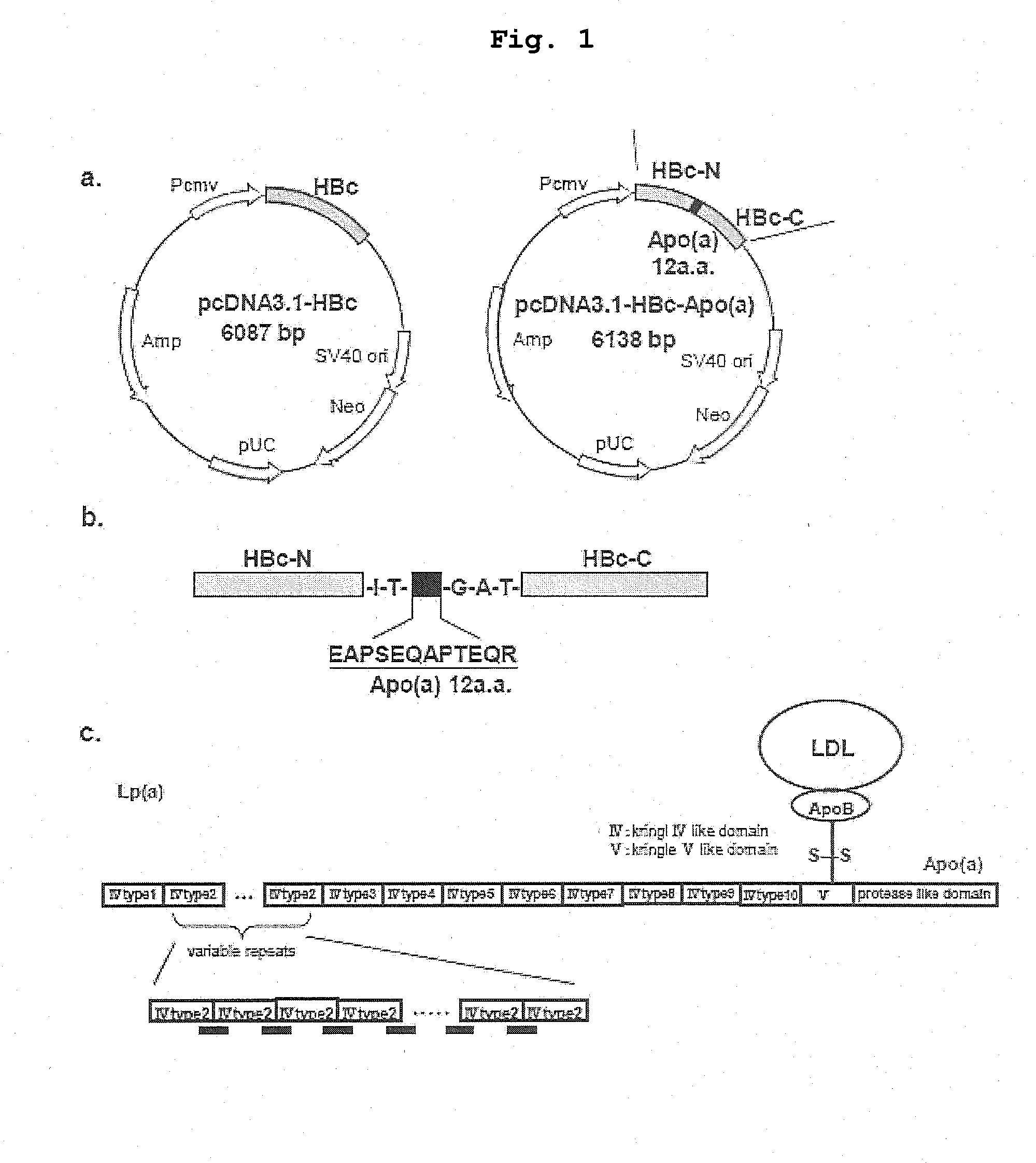
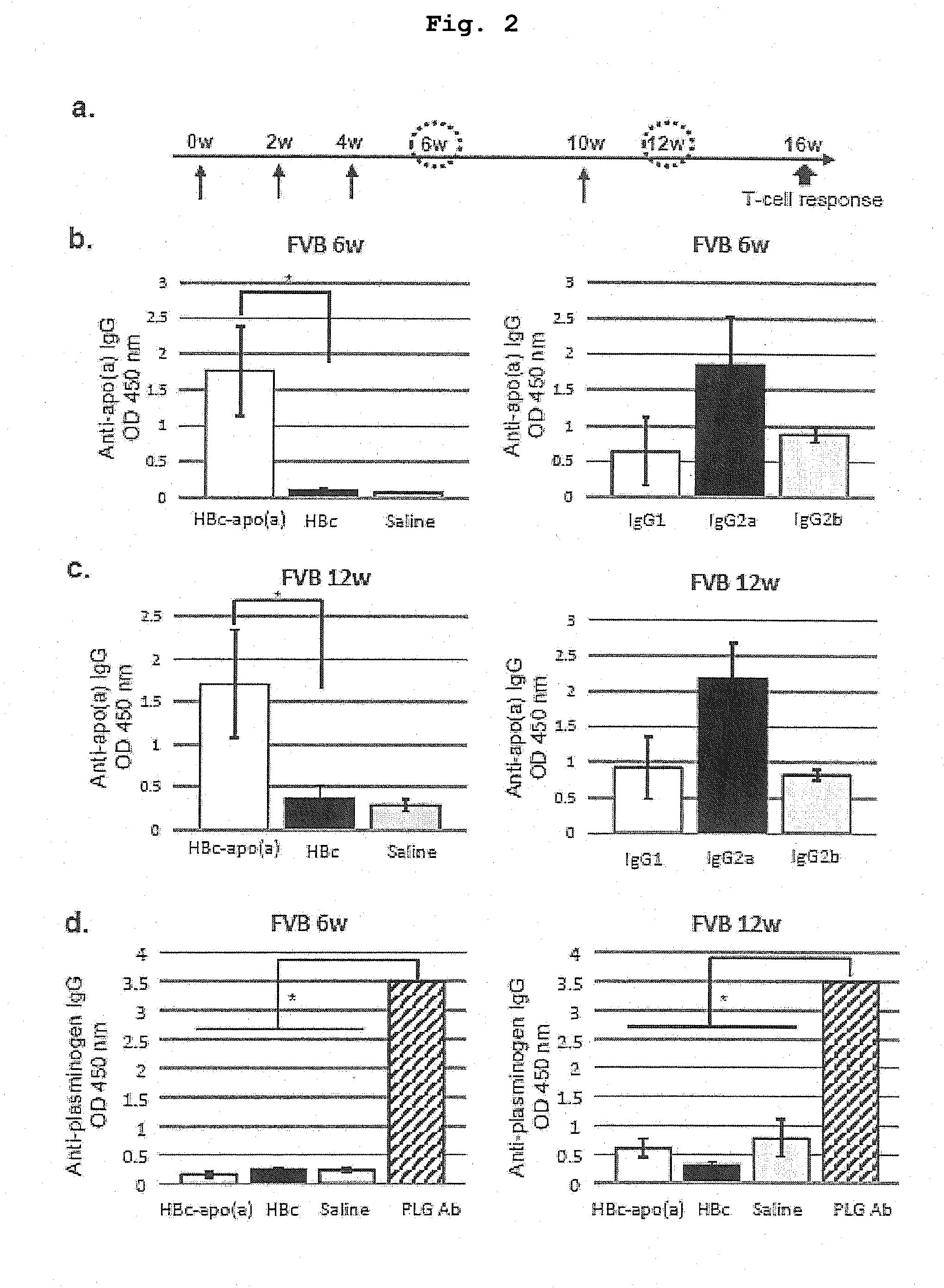
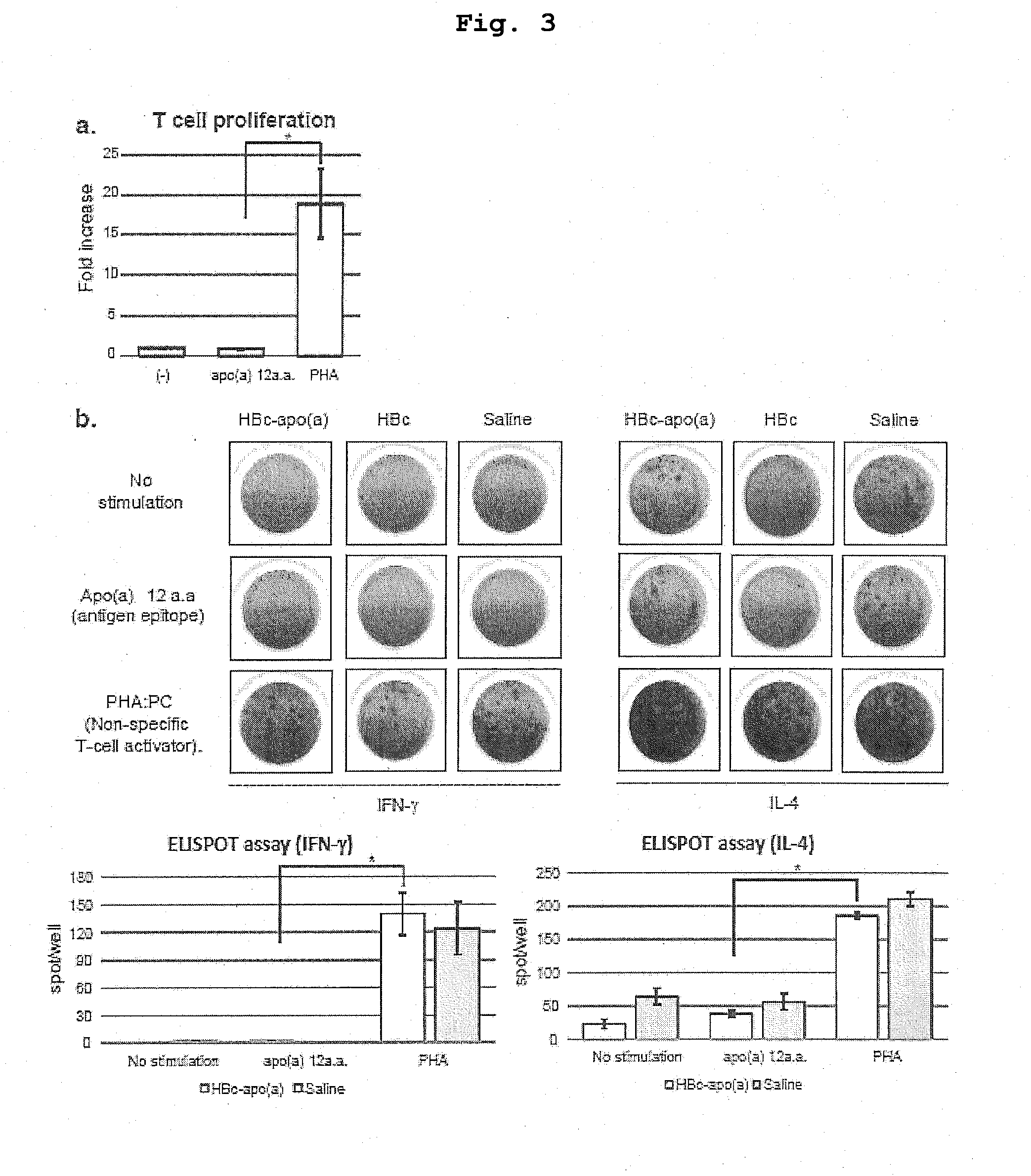
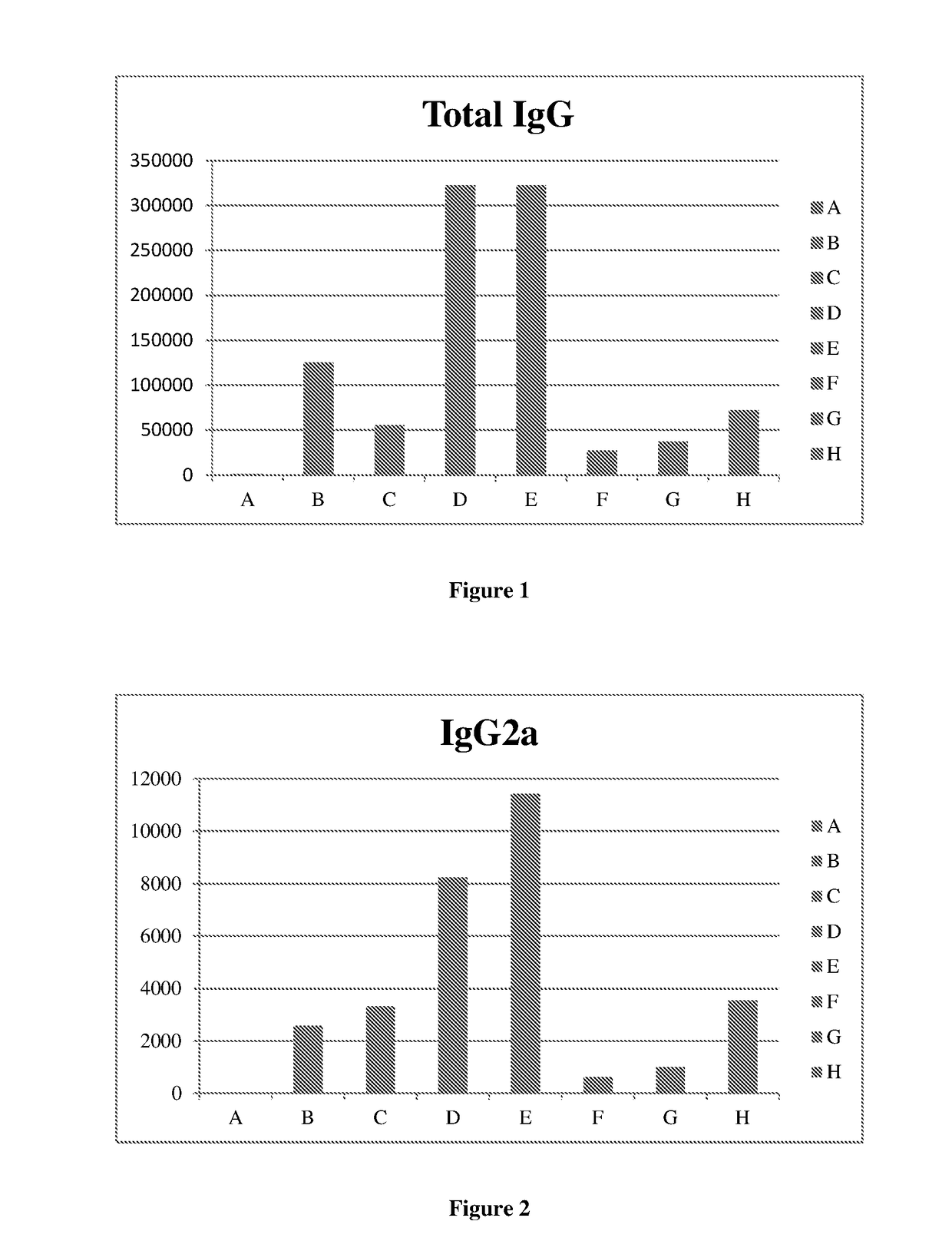
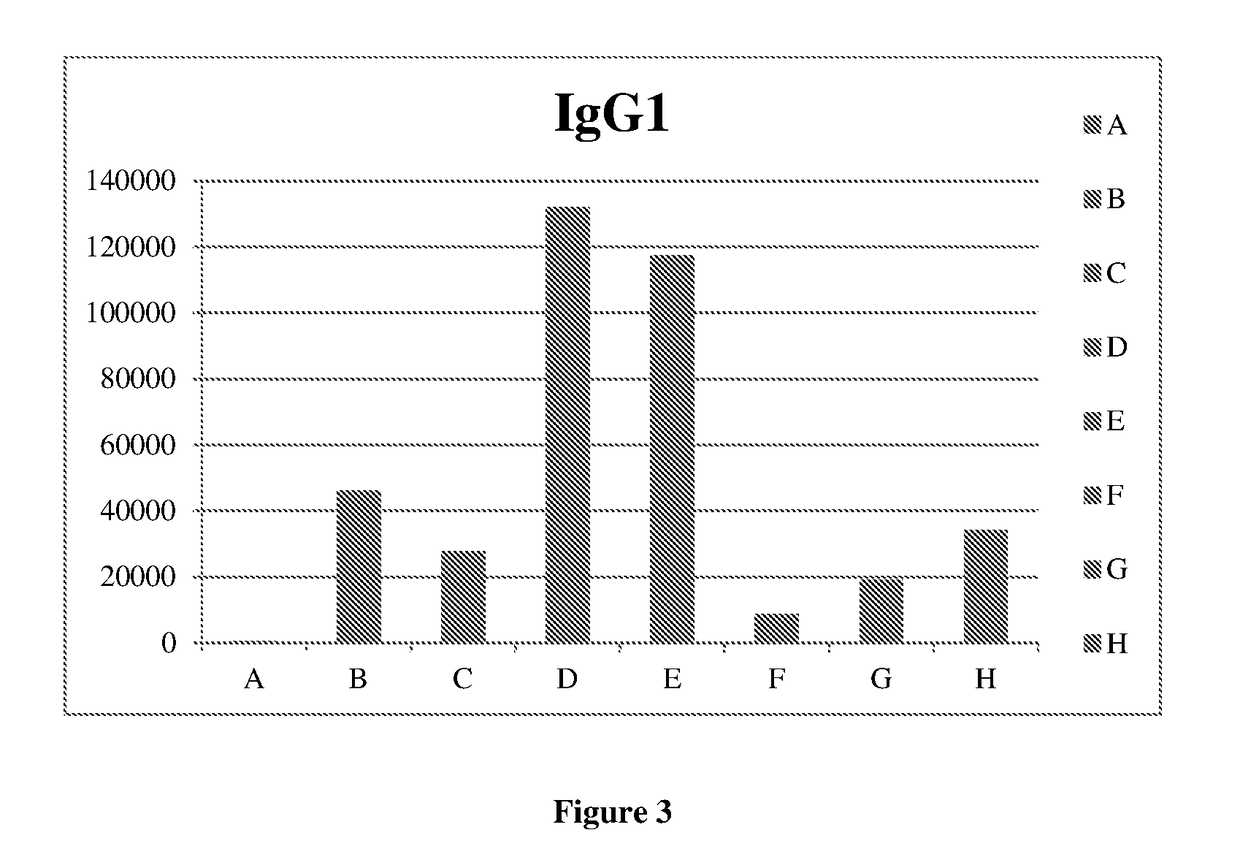
DNA VACCINE CONTAINING SPECIFIC EPITOPE OF APOLIPOPROTEIN (a)
InactiveUS20140086944A1Treating and preventing arteriosclerosisAvoid inductionApolipeptidesViral antigen ingredientsEpitopeHepatitis B virus core Antigen
The present invention provides an agent for the treatment or prophylaxis of arteriosclerosis comprising an expression vector encoding a chimeric Hepatitis B virus core antigen polypeptide inserted with an amino acid sequence containing a specific epitope of apolipoprotein (a), wherein the amino acid sequence containing the specific epitope is inserted between the amino acid residues 80 and 81 of the hepatitis B virus core antigen polypeptide.
Owner:ANGES MG INC
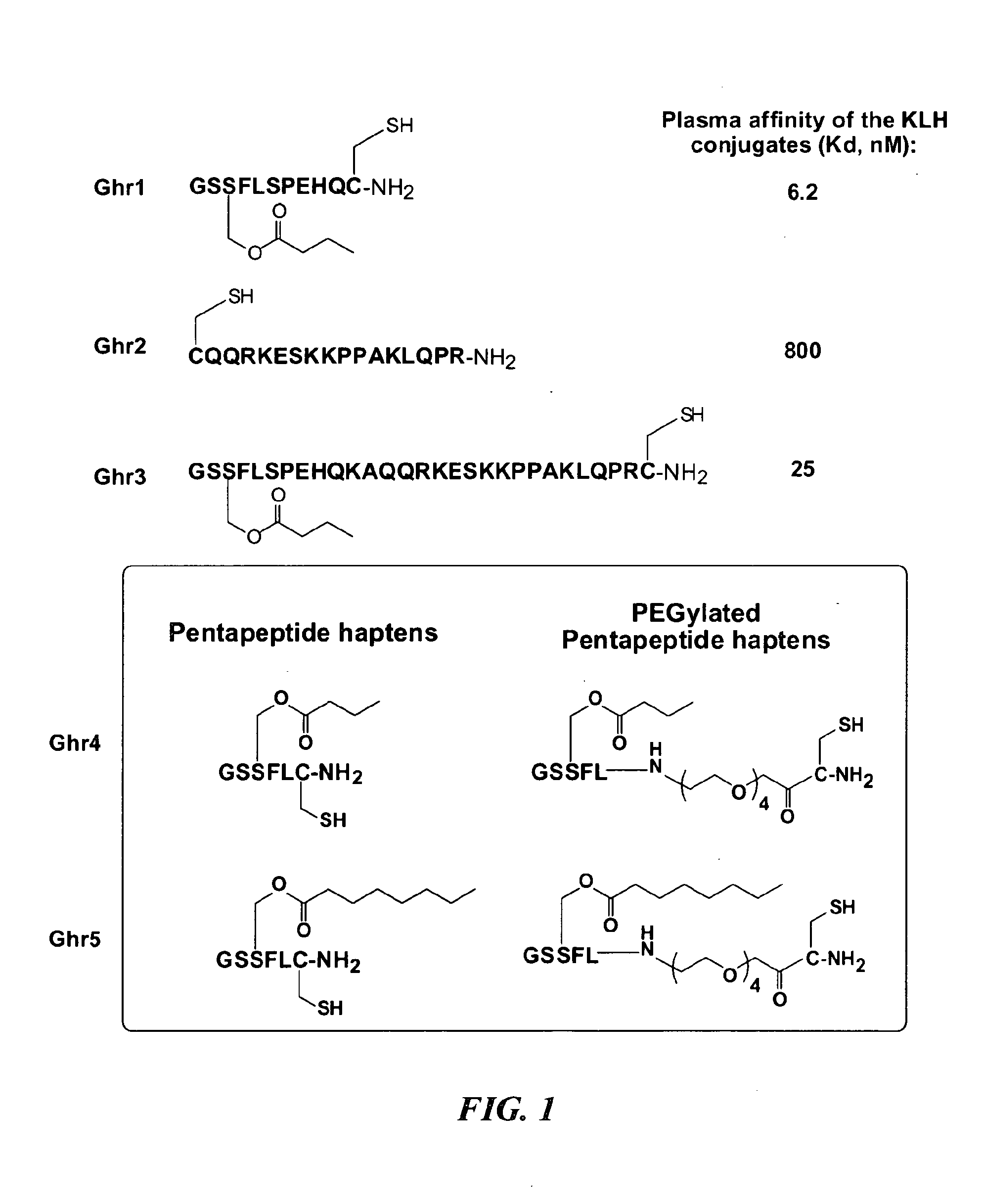
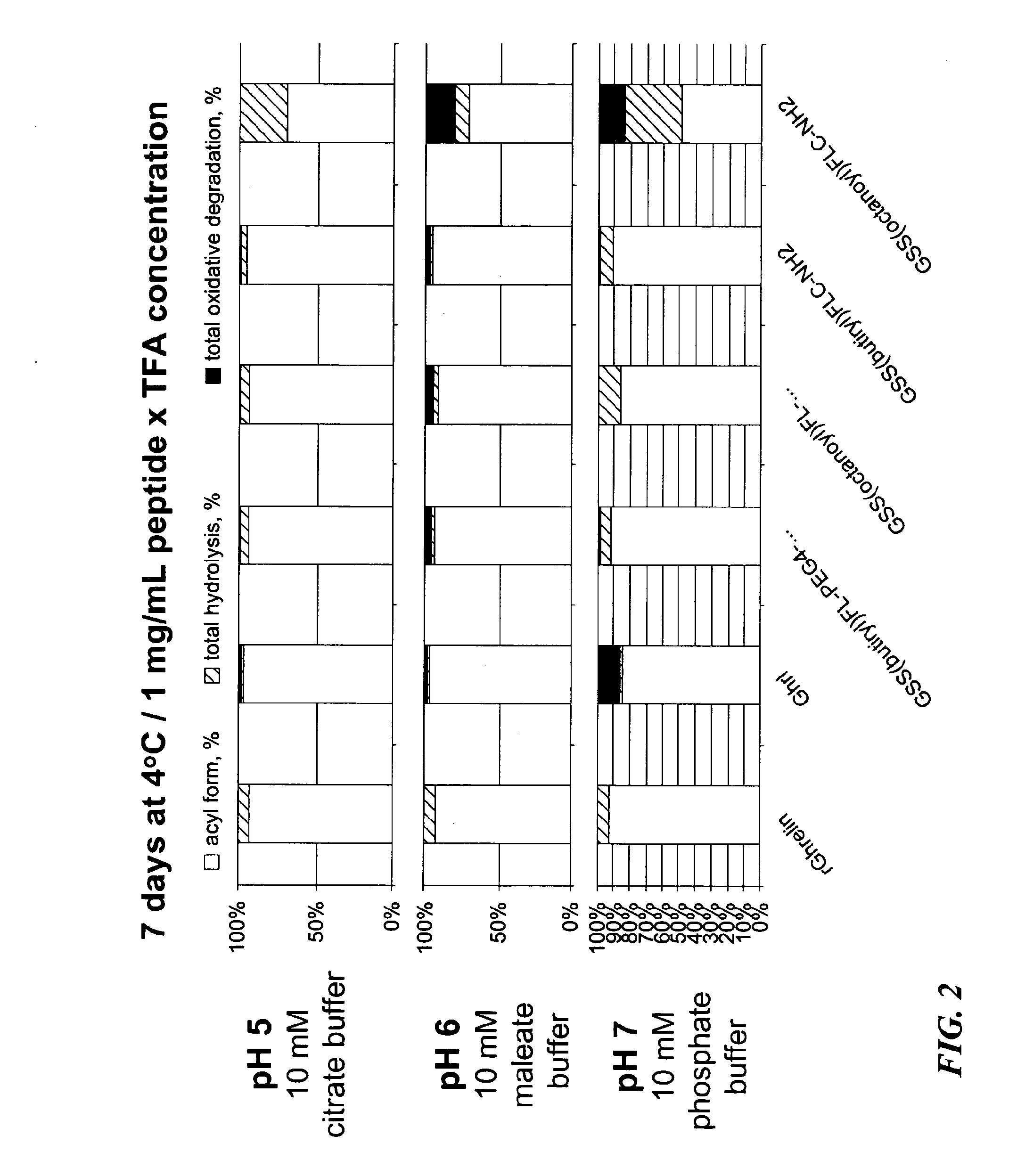
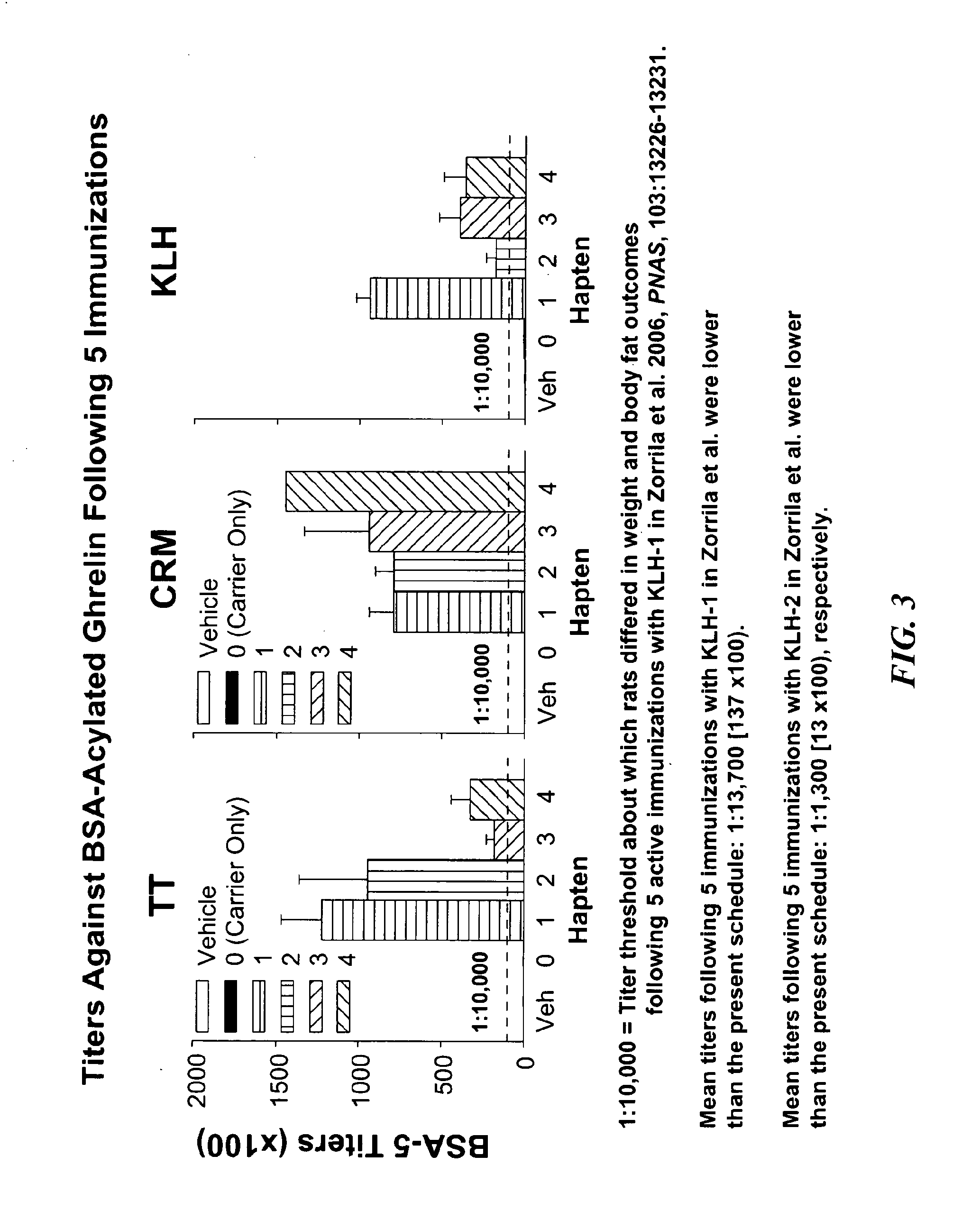
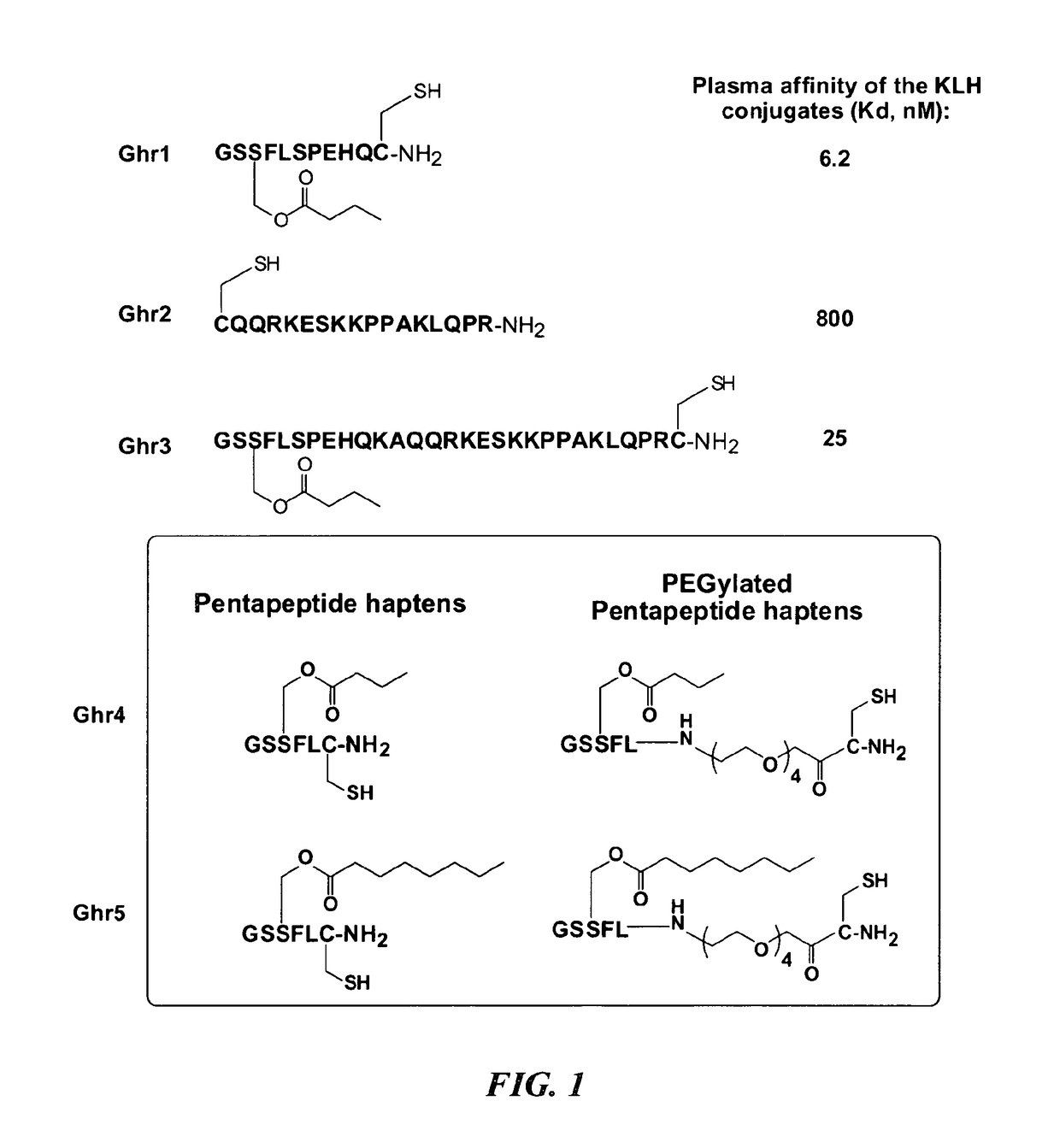
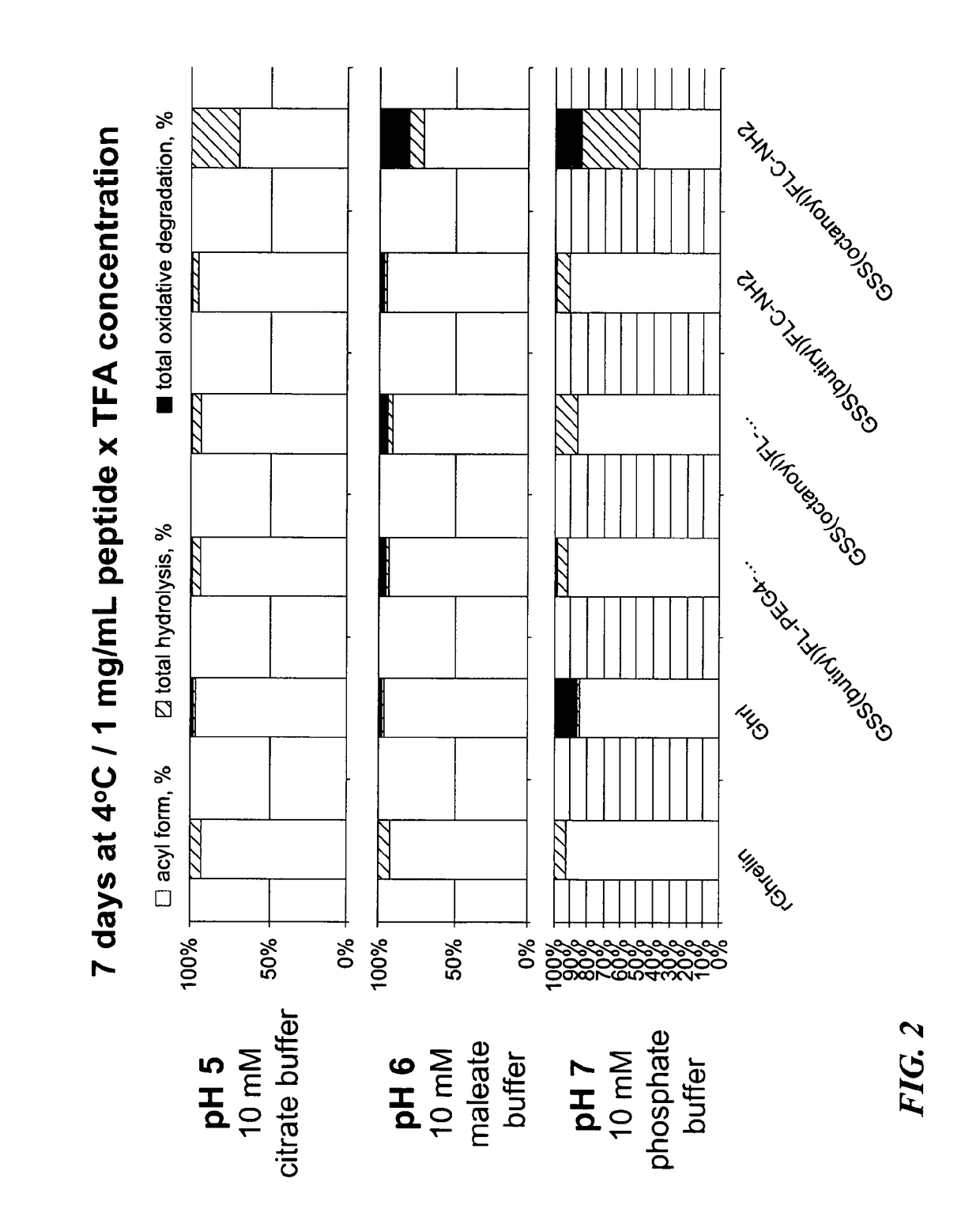
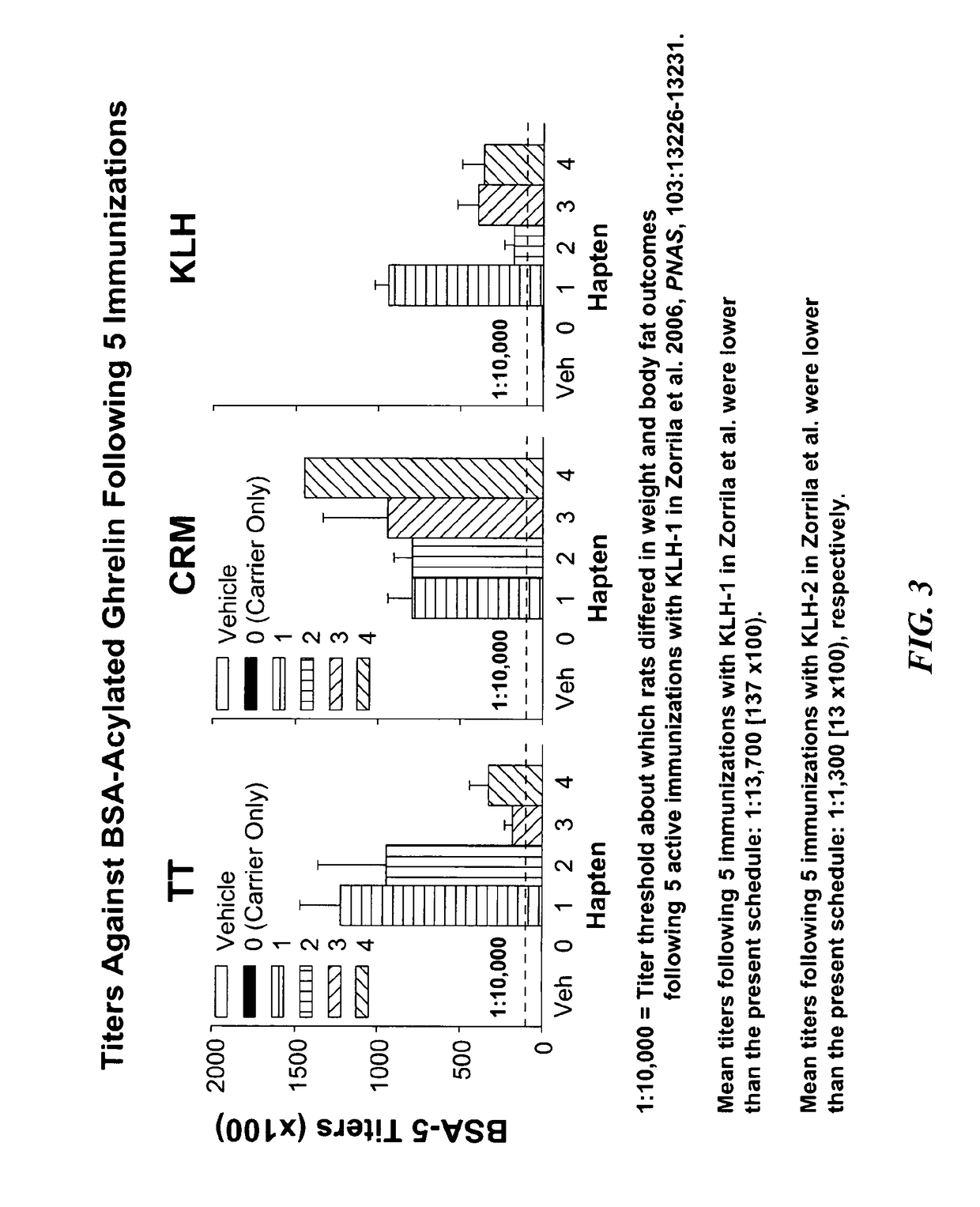
Ghrelin Mimetic Polypeptide Hapten Immunoconjugates Having Improved Solubility and Immunogenicity and Methods of Use Thereof
ActiveUS20140086949A1Regulate energy balanceEase weight gainObesity gene productsBacterial antigen ingredientsGrowth hormonePolyethylene glycol
Immunoconjugates for impeding weight gain and treating obesity in a subject are disclosed. The immunoconjugates comprise a ghrelin mimetic polypeptide hapten, a spacer moiety comprising one of more polyethylene glycol (PEG) units, and a protein carrier moiety. Immunoconjugates optionally include a conjugation moiety for conjugating the polypeptide hapten with a linker moiety or the protein carrier moiety and a linker moiety for conjugating the conjugation moiety with the protein carrier moiety.
Owner:THE SCRIPPS RES INST
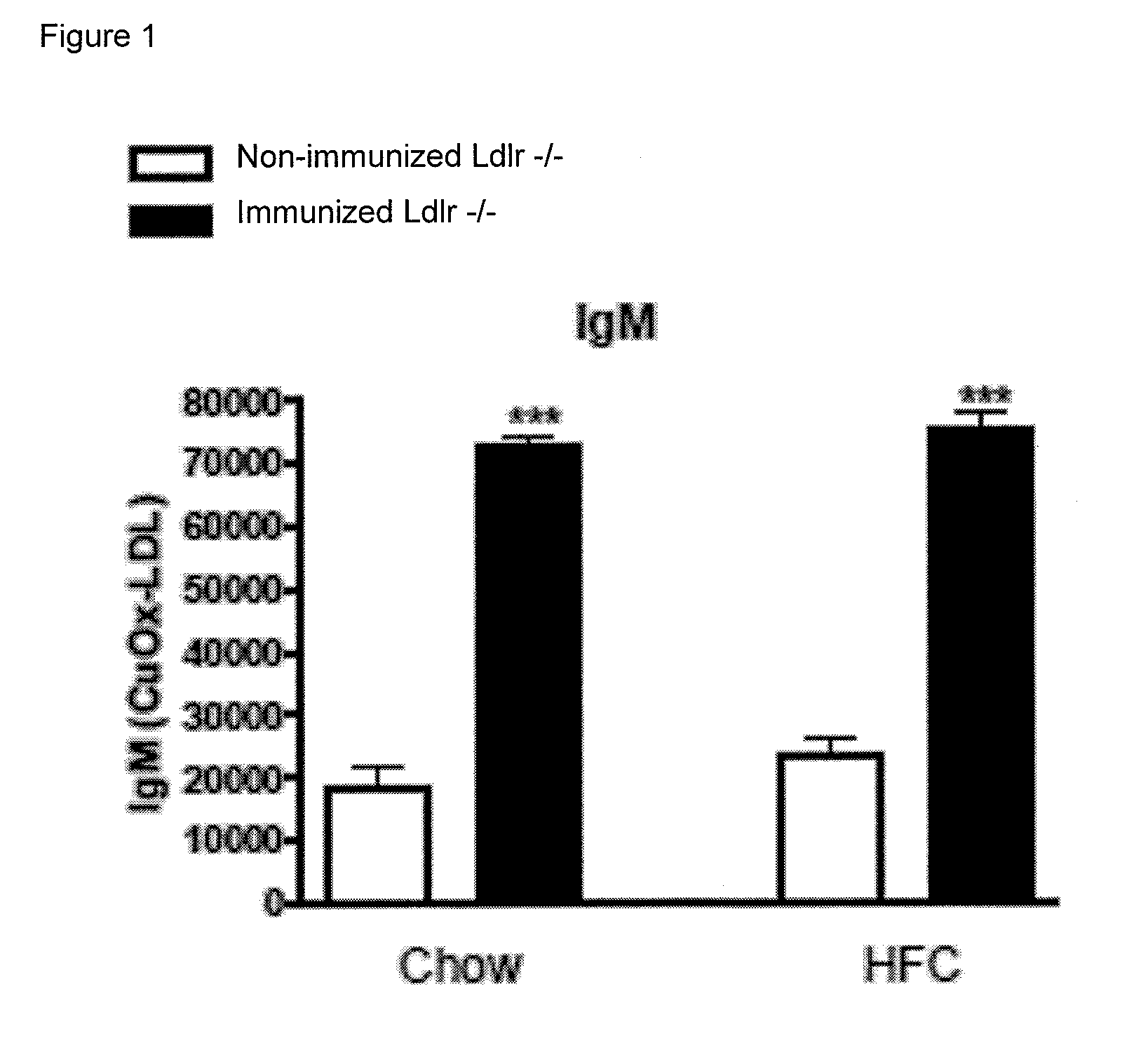
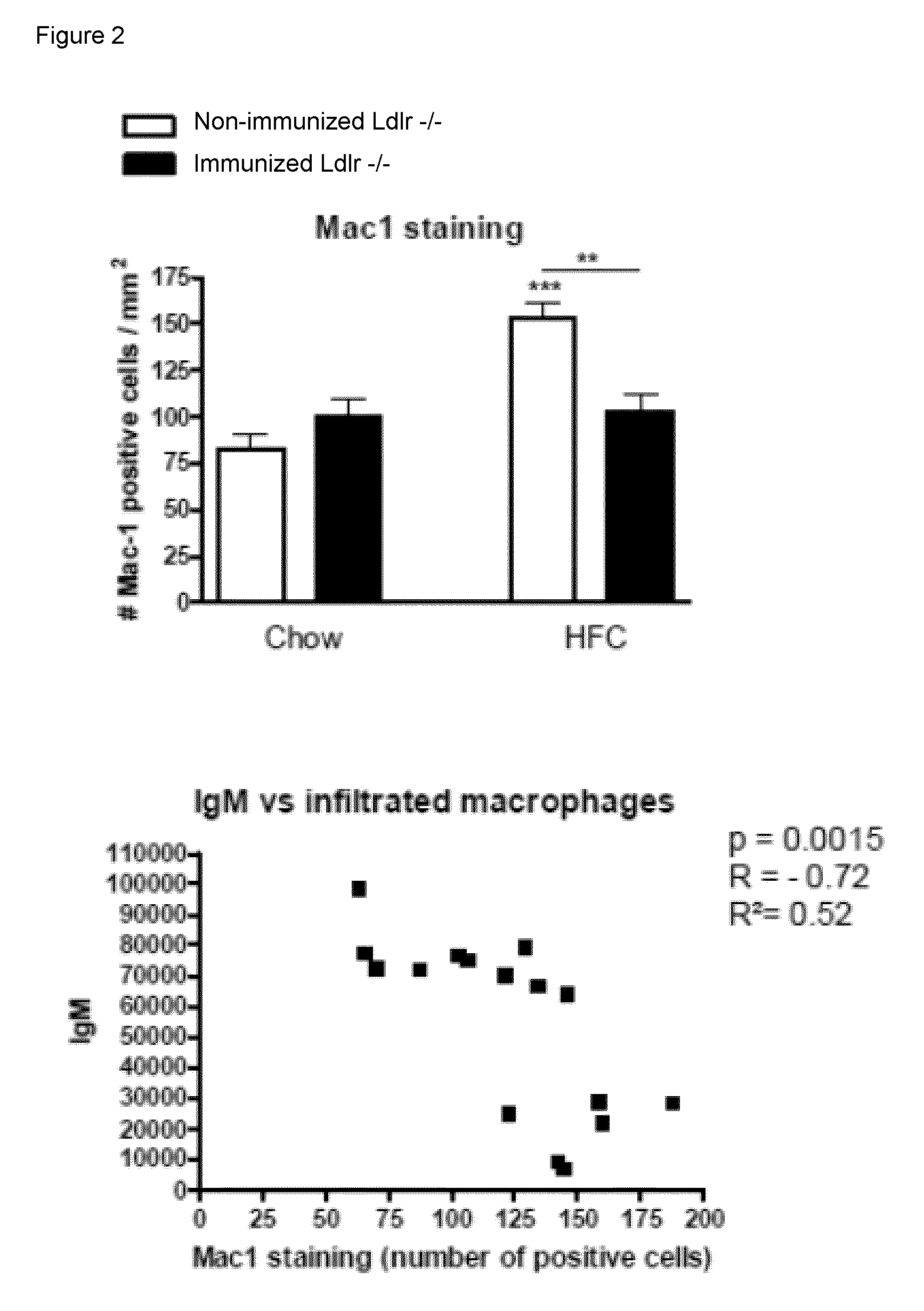
Methods for treating liver inflammation in a subject suffering from non-alcoholic steatohepatitis
ActiveUS9393303B2Improve the level ofUseful in treatingBacterial antigen ingredientsDigestive systemHepatic inflammationIn vivo
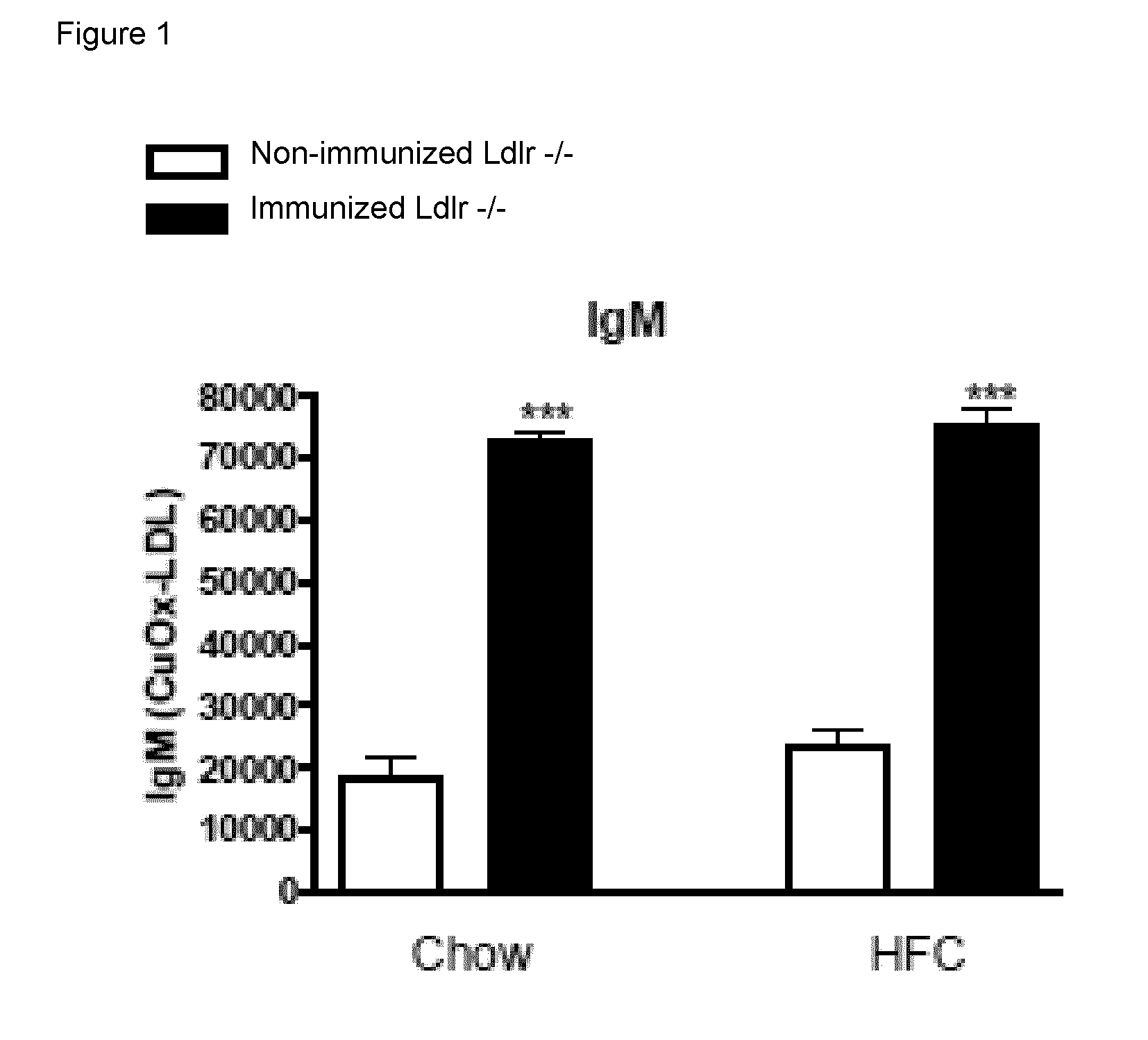
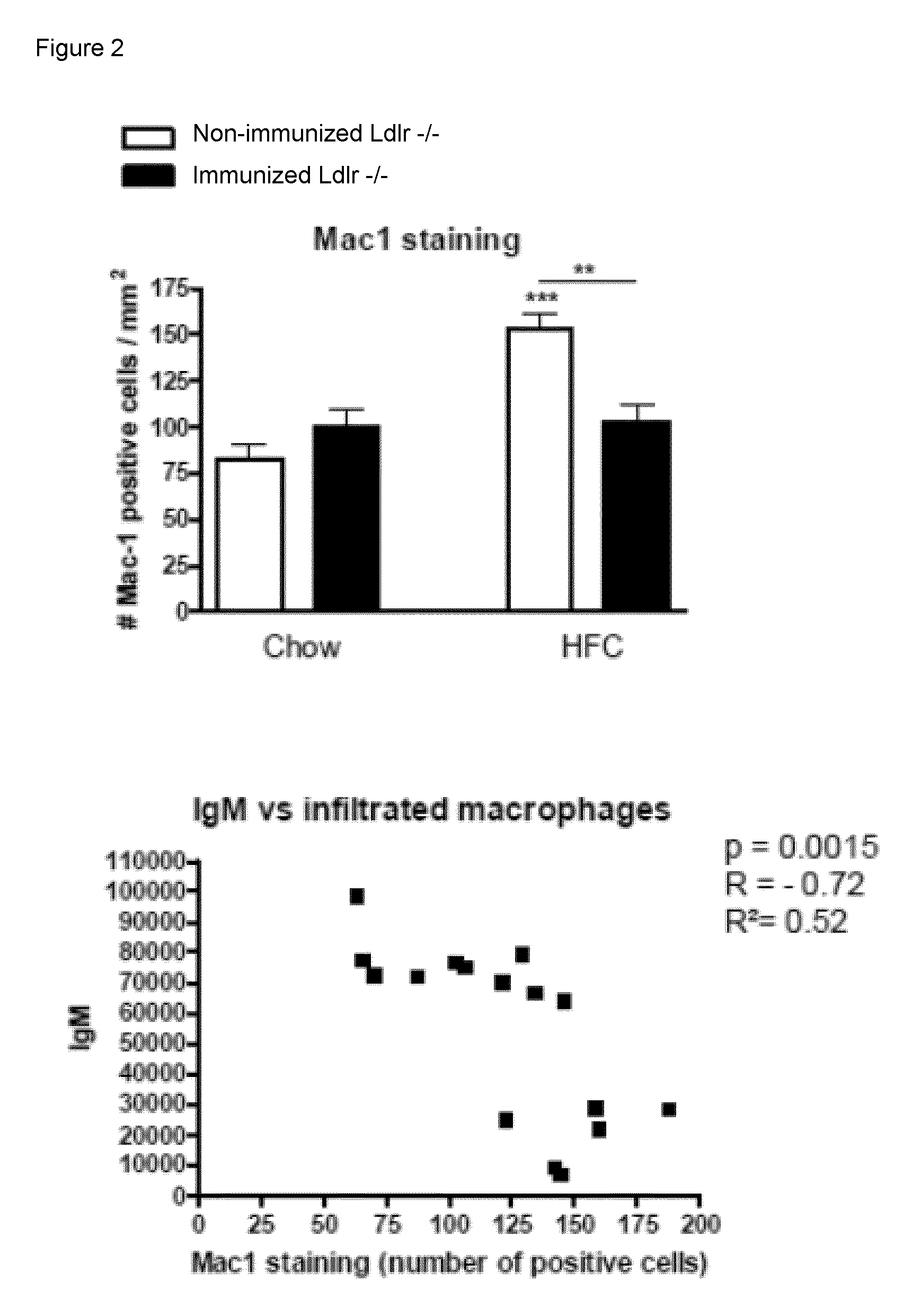
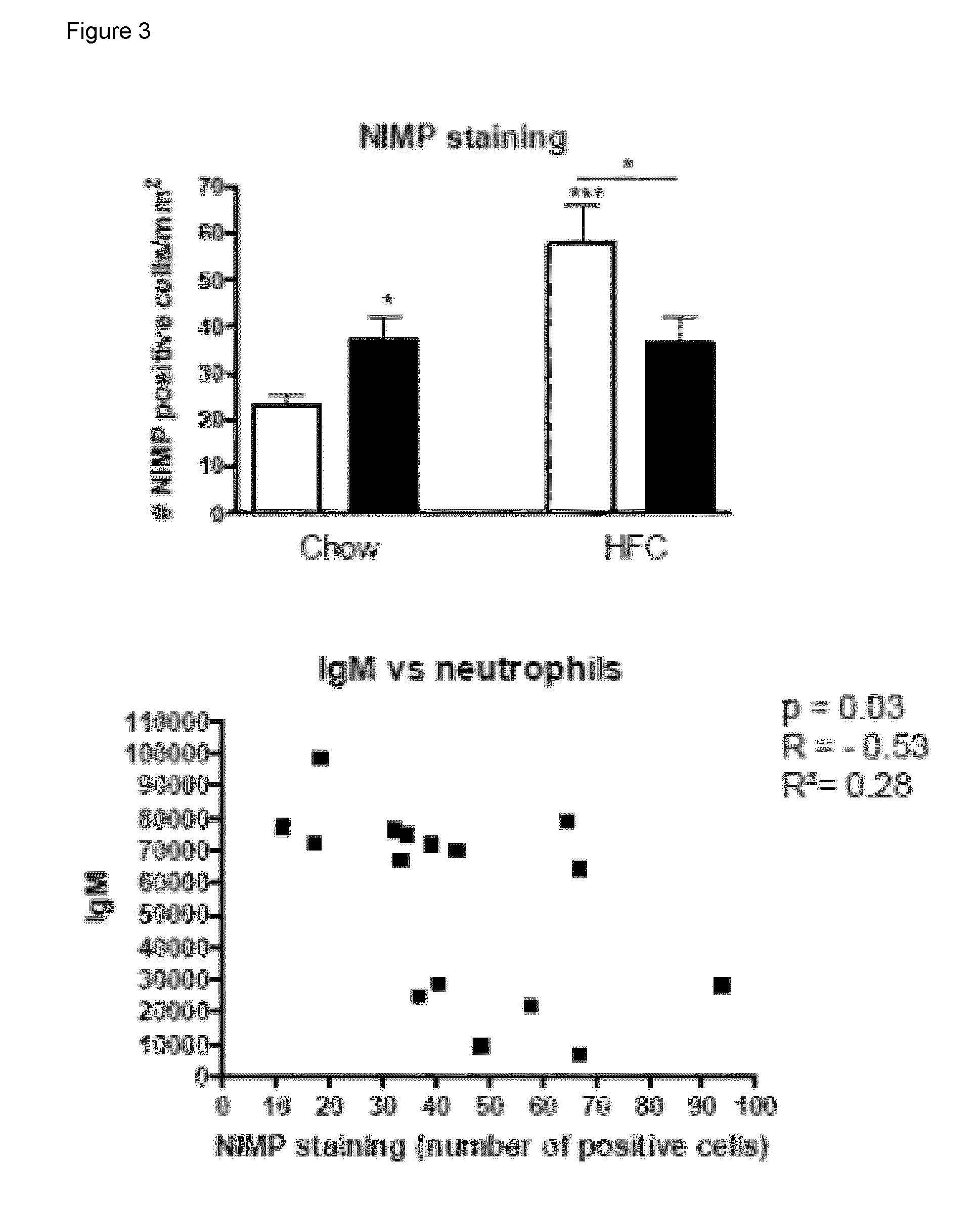
The invention is in the field of prevention and medical treatment of liver diseases, in particular non-alcoholic steatohepatitis (NASH). The invention provides means and methods for the treatment of hepatic inflammation, fibrosis and more in particular NASH. More in particular, the invention provides a composition capable of raising anti-oxLDL antibodies in vivo for use in the treatment of liver inflammation or fibrosis.
Owner:MAASTRICHT UNIVERSITY +1
Virus like particle with efficient epitope display
ActiveUS20190022206A1Effective displayProtective immunityBacterial antigen ingredientsLipid/lipoprotein ingredientsDiseaseEpitope
The invention relates to a virus like particle (VLP) based vaccine. The virus-like particle constitutes a non-naturally occurring, ordered and repetitive antigen array display scaffold which can obtain a strong and long-lasting immune response in a subject. The VLP based vaccine may be used for the prophylaxis and / or treatment of a disease including, but is not limited to, cancer, cardiovascular, infectious, asthma, and / or allergy diseases / disorders.
Owner:UNIVERSITY OF COPENHAGEN
Method for treating liver inflammation, fibrosis and non-alcoholic steatohepatitis
ActiveUS20140044734A1Improve the level ofUseful in treatingBacterial antigen ingredientsDigestive systemHepatic inflammationIn vivo
The invention is in the field of prevention and medical treatment of liver diseases, in particular non-alcoholic steatohepatitis (NASH). The invention provides means and methods for the treatment of hepatic inflammation, fibrosis and more in particular NASH. More in particular, the invention provides a composition capable of raising anti-oxLDL antibodies in vivo for use in the treatment of liver inflammation or fibrosis.
Owner:MAASTRICHT UNIVERSITY +1
Compositions and methods for modified dendrimer nanoparticle delivery
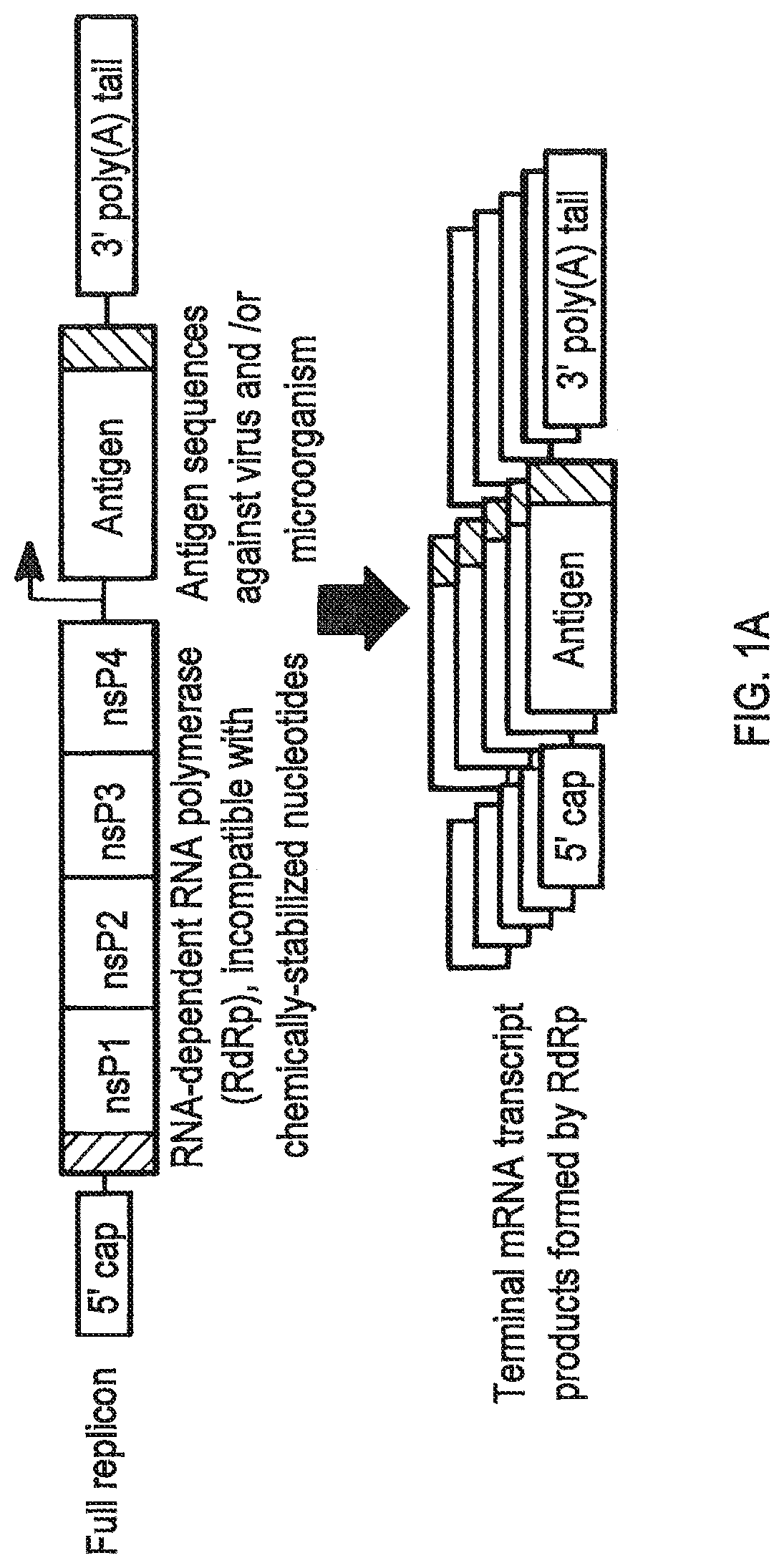
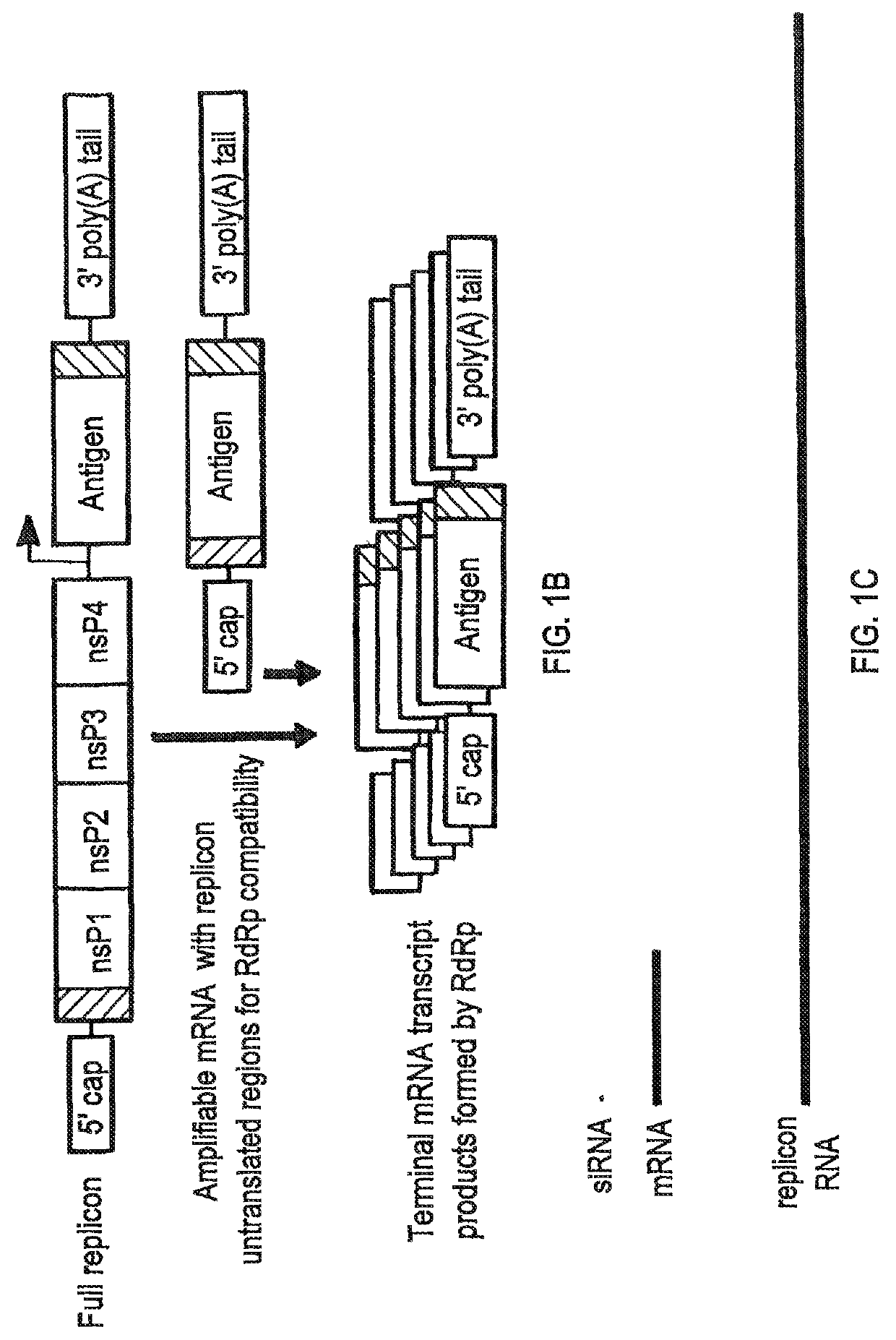
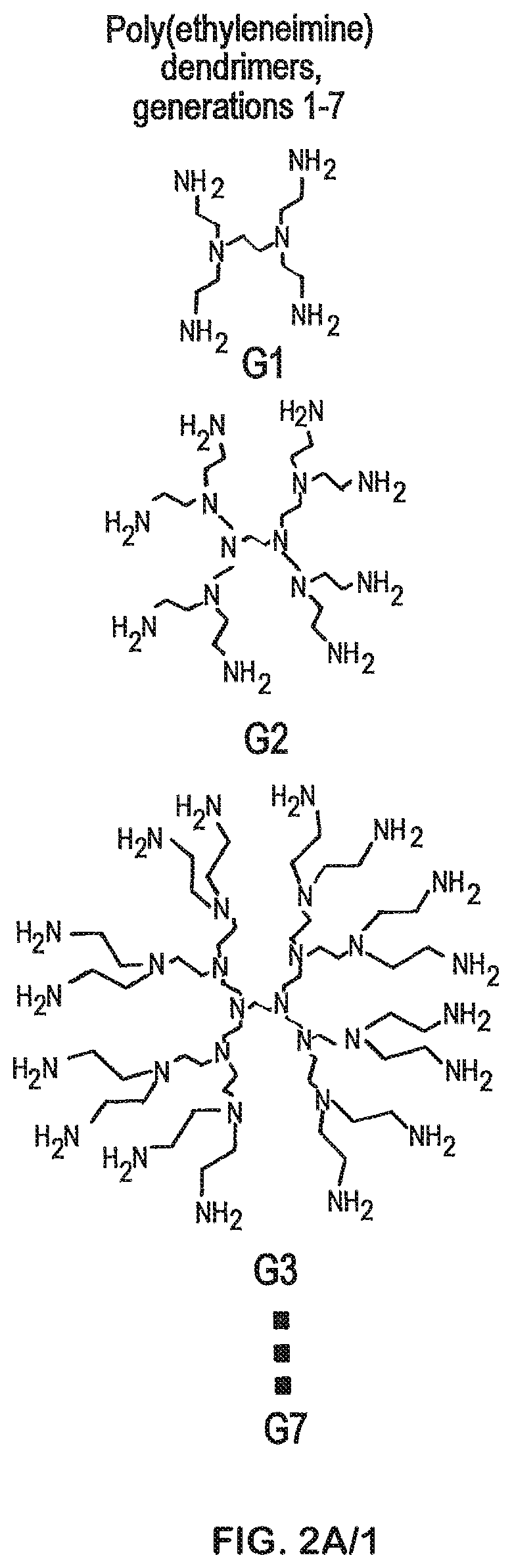
Compositions and methods for modified dendrimer nanoparticle (“MDNP”) delivery of therapeutic, prophylactic and / or diagnostic agent such as large repRNA molecules to the cells of a subject have been developed. MDNPs efficiently drive proliferation of antigen-specific T cells against intracellular antigen, and potentiate antigen-specific antibody responses. MDNPs can be multiplexed to deliver two or more different repRNAs to modify expression kinetics of encoded antigens and to simultaneous deliver repRNAs and mRNAs including the same UTR elements that promote expression of encoded antigens.
Owner:MASSACHUSETTS INST OF TECH +1
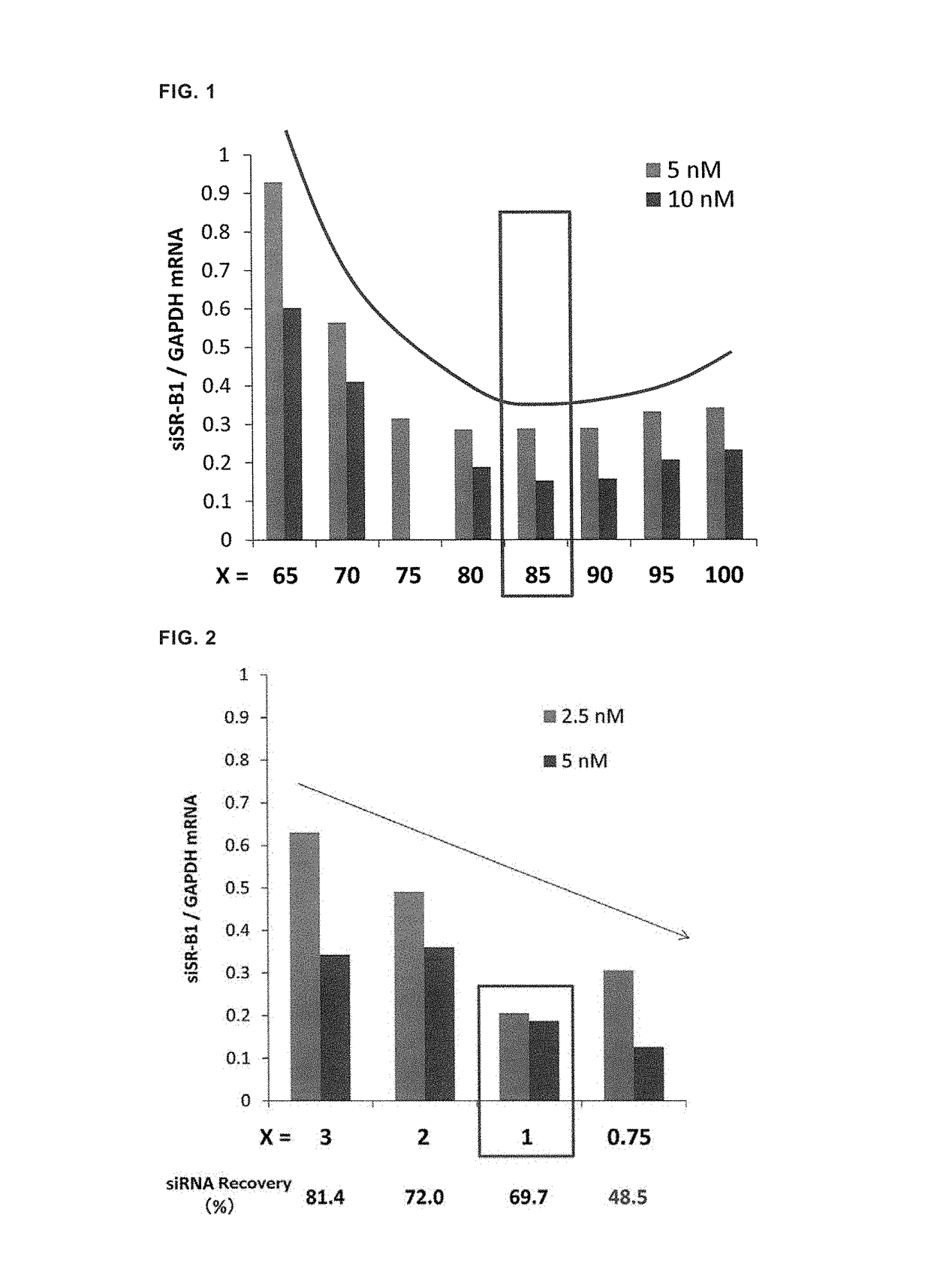
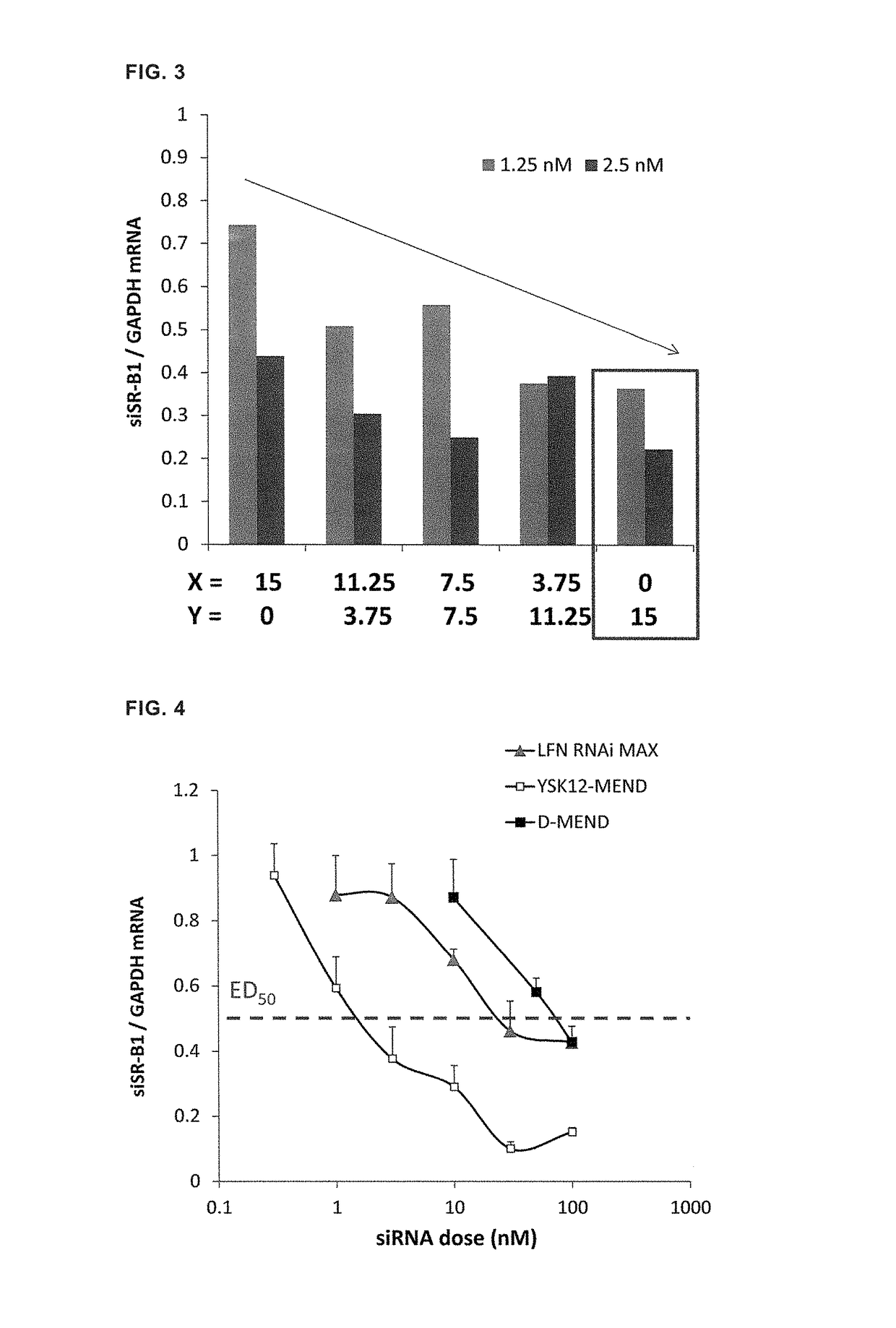
Lipid membrane structure for intracellular delivery of siRNA
ActiveUS10182987B2Efficient releaseEfficient deliveryOrganic active ingredientsOrganic chemistryLipid formationChemistry
A lipid membrane structure encapsulating an siRNA inside thereof and containing a lipid compound of the formula (I) as a lipid component (R1 and R2 represent CH3—(CH2)n—CH═CH—CH2—CH═CH—(CH2)m—, n represents an integer of 3 to 5, m represents an integer of 6 to 10, p represents an integer of 2 to 7, and R3 and R4 represent a C1-4 alkyl group or a C2-4 alkenyl group.
Owner:HOKKAIDO UNIVERSITY
Method of treating lung cancer by vaccination with MUC-1 lipopeptide
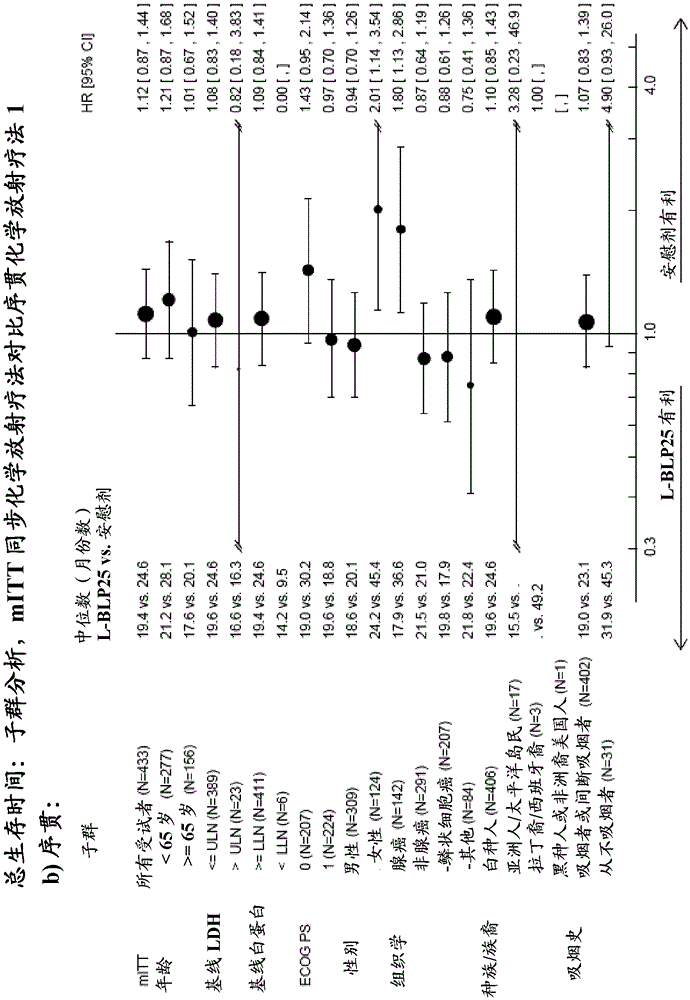
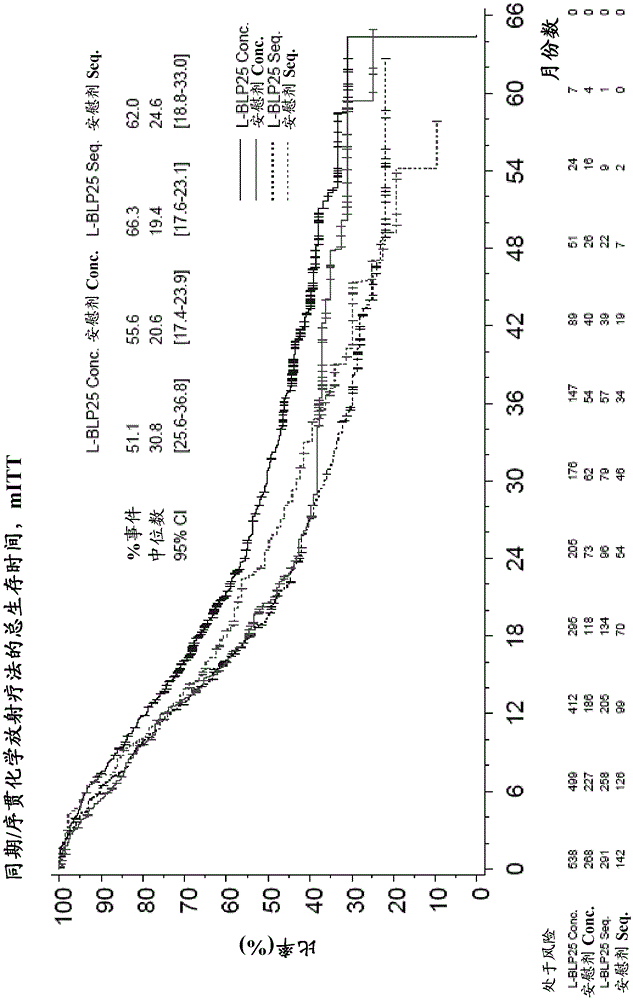
InactiveCN105283199AHeavy metal active ingredientsOrganic active ingredientsVaccinationTreatment of lung cancer
The invention is directed to the treatment of lung cancer, preferably non-small cell lung cancer (NSCLC) by means of a combination therapy comprising concurrent chemo-radiotherapy followed by vaccination with a muc-1 lipopetide. The therapy elicits prolonged survival rates compared to a respective therapy including sequential chemo-radiotherapy.
Owner:MERCK PATENT GMBH
Apolipoprotein E as an adjuvant for lipid antigens
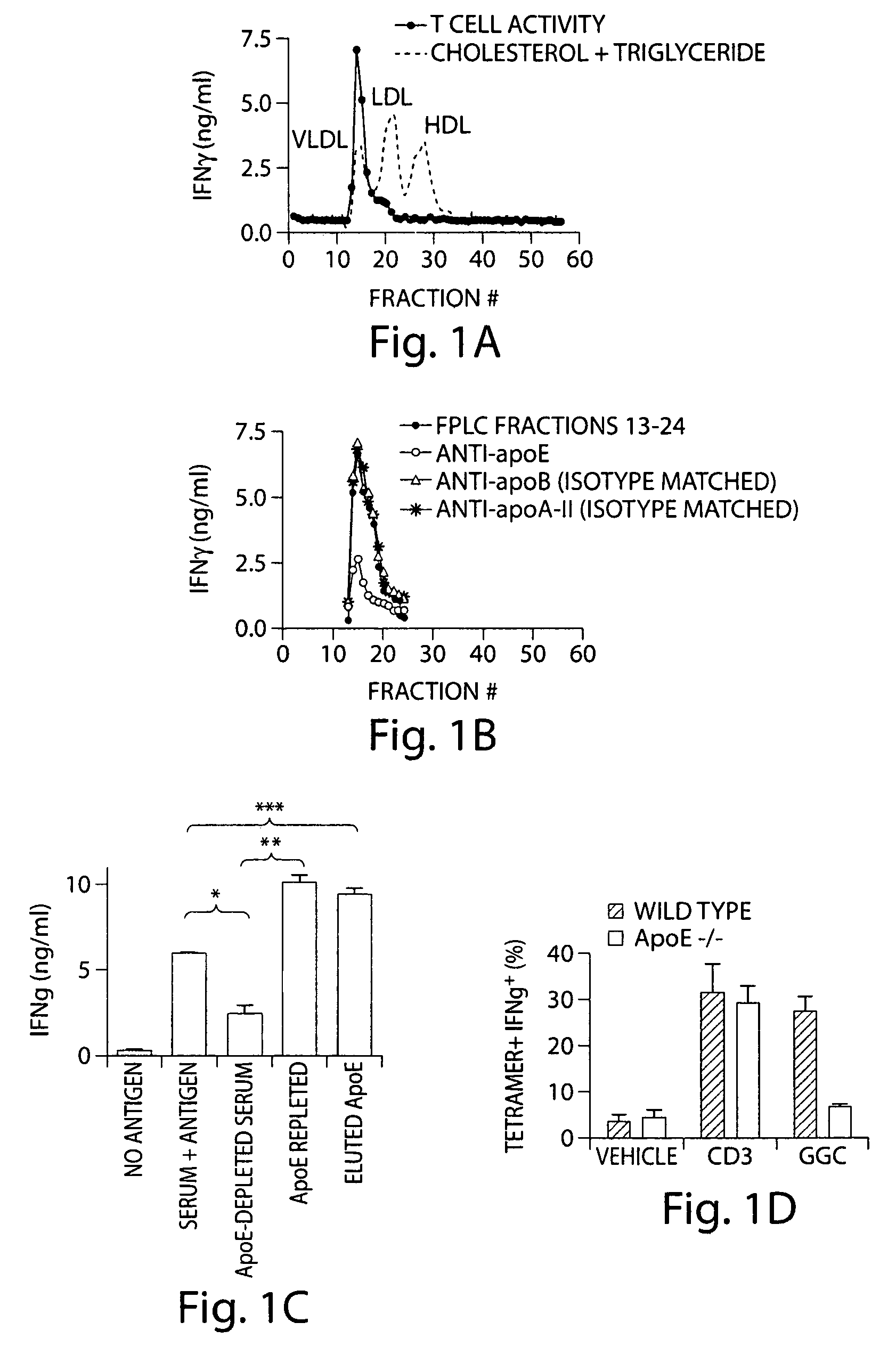
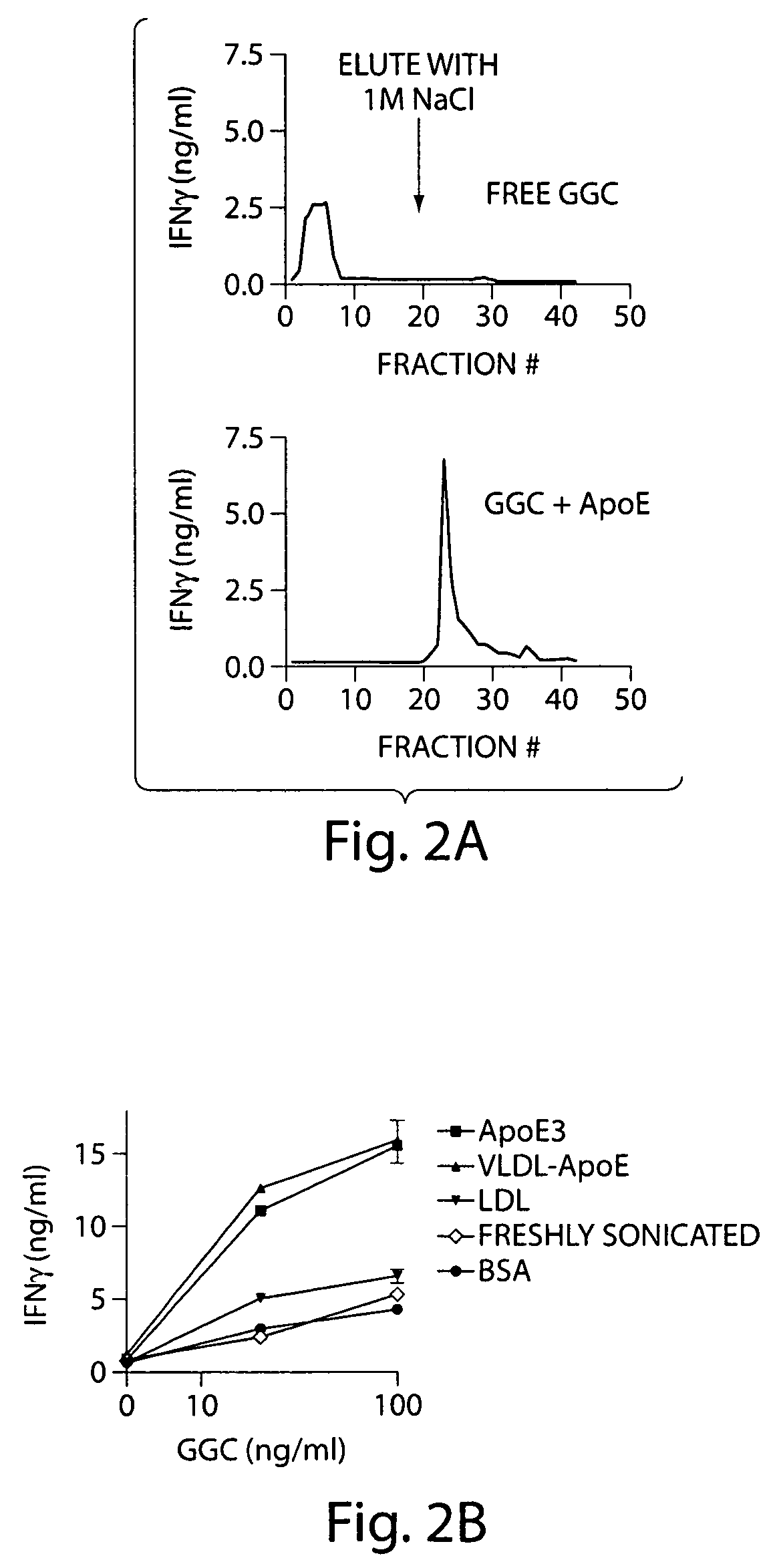
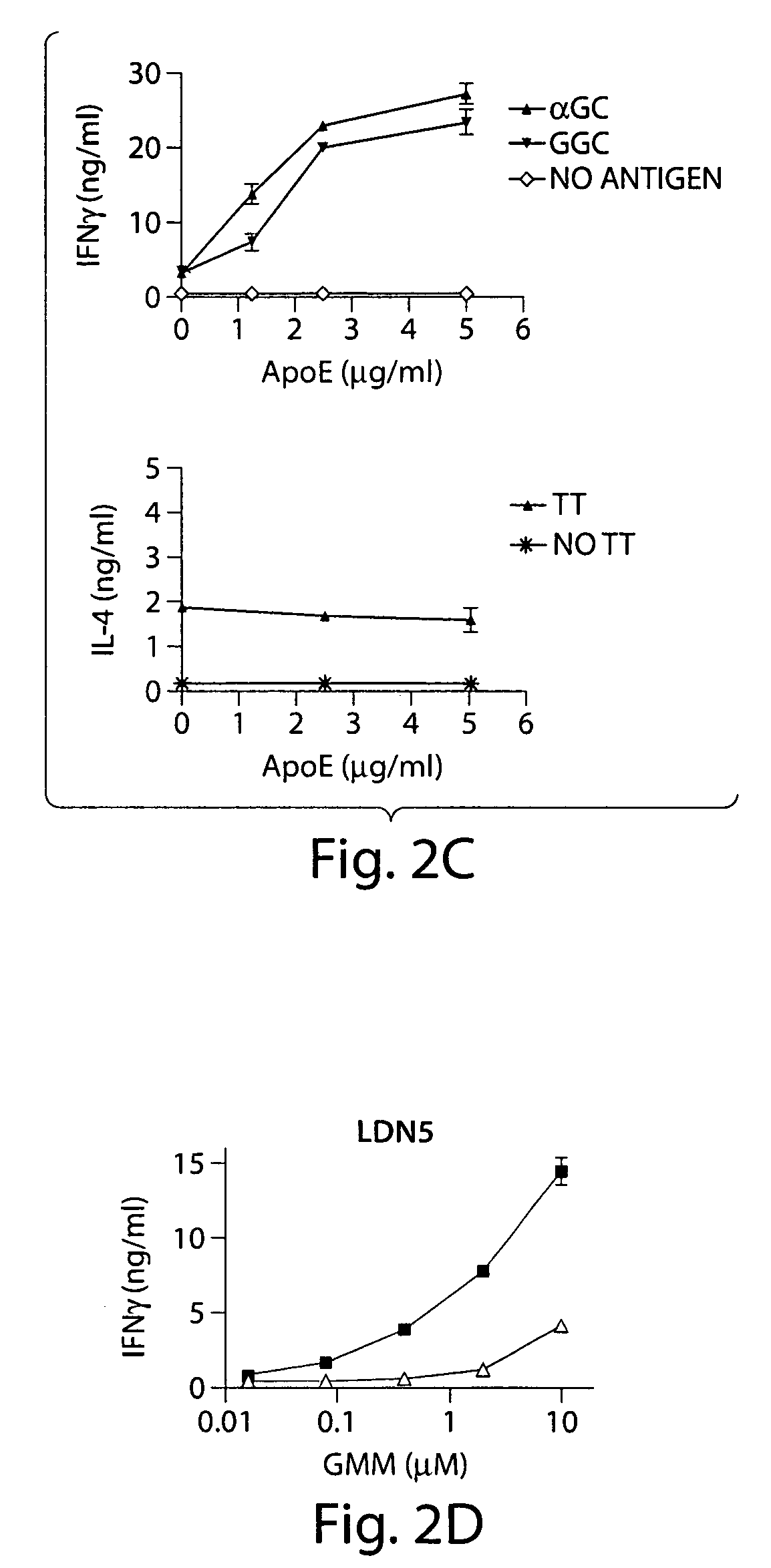
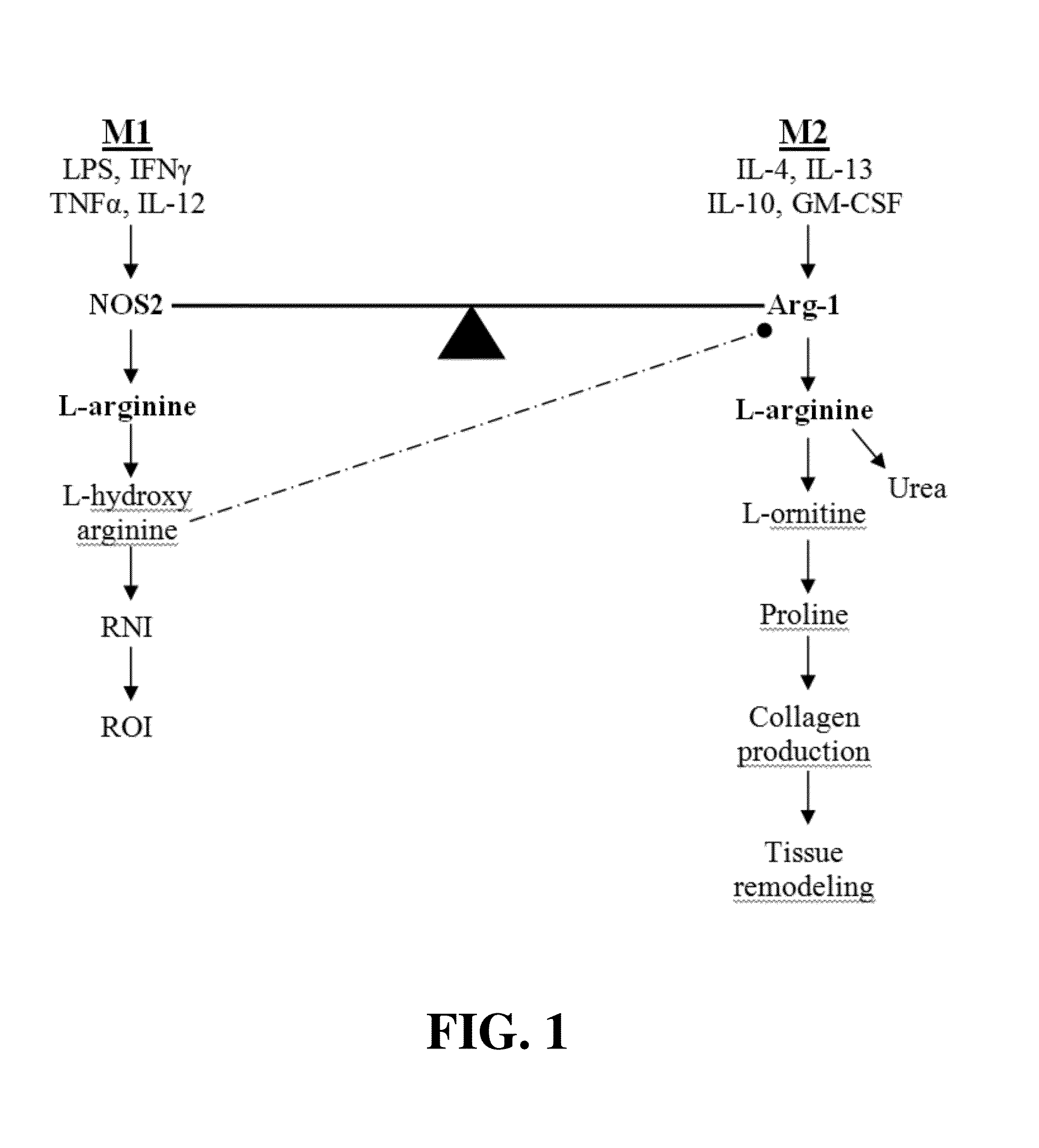
InactiveUS7608246B2Stimulate immune responseFacilitating the uptake of lipidsAntibacterial agentsOrganic active ingredientsLipid formationApolipoprotein e4
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Substances, vaccines and methods for diagnosing and reducing incidences of transplant rejection
ActiveUS20150233941A1Reduce the populationReduce morbidityLipid/lipoprotein ingredientsDisease diagnosisNatural antibodyTransplant rejection
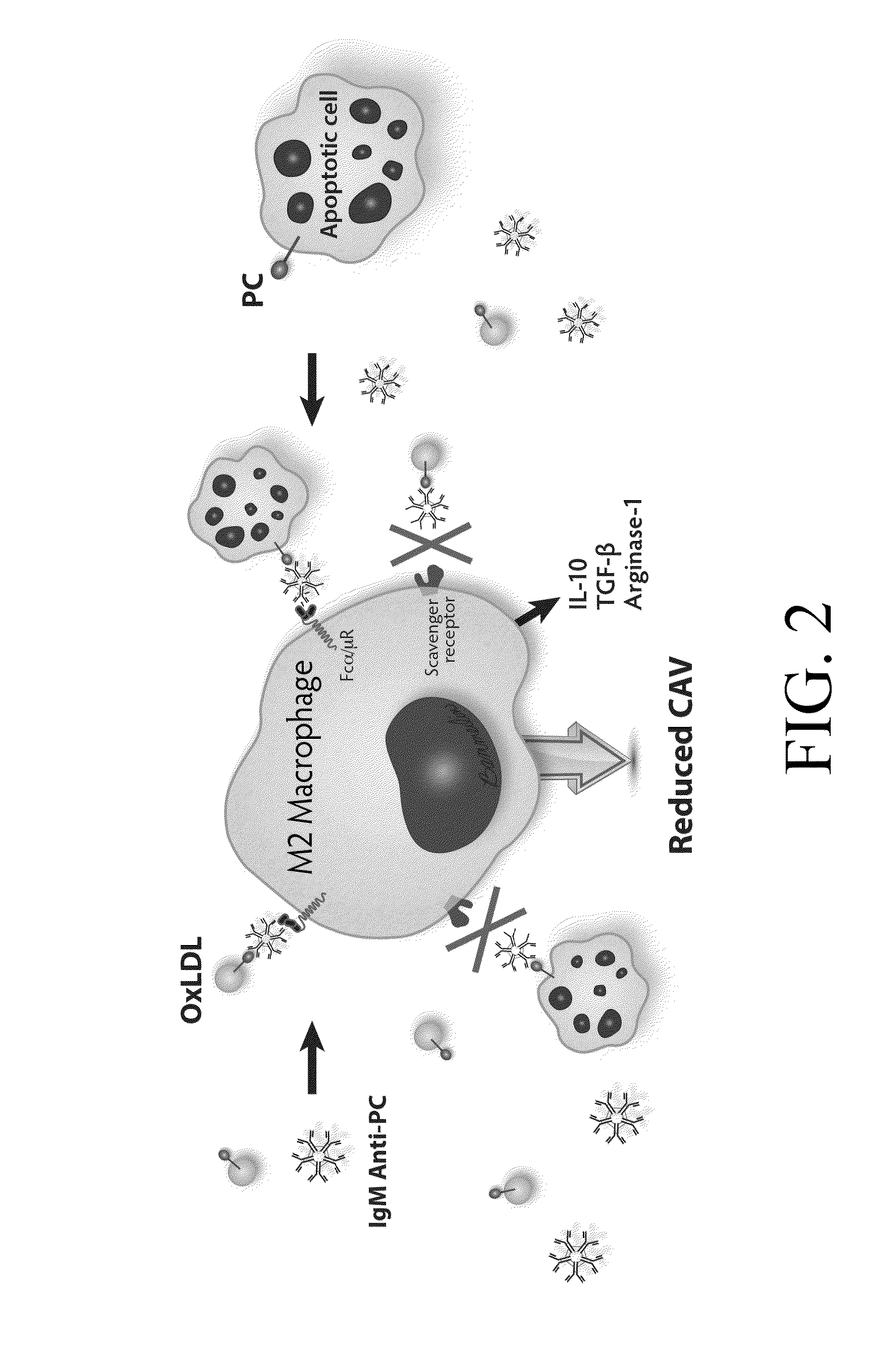
Methods and products for diagnosing, treating and / or delaying onset of chronic allograft rejection, including cardiac allograft vasculopathy. The method for screening an allograft recipient (including pregnant women) for chronic allograft rejection comprises the steps of measuring an amount of a natural antibody within a biological sample and comparing the amount of the first natural antibody with the amount of the first natural antibody present within a control sample; wherein a decrease in the amount of the first natural antibody as compared to those levels seen in the control indicates a diagnosis of being at-risk for or experiencing chronic allograft rejection. Furthermore, in an embodiment of a composition for preventing or treating chronic allograft rejection, the composition comprises a therapeutically effective amount of phosphorylcholine sufficient to initiate the production of anti-phoshporylecholine natural antibodies in a mammal following administration thereto.
Owner:3DT HLDG
LIPID MEMBRANE STRUCTURE FOR siRNA INTRACELLULAR DELIVERY
ActiveUS20170273905A1Efficient releaseEfficient deliveryOrganic active ingredientsOrganic chemistryMembrane structureChemistry
A lipid membrane structure encapsulating an siRNA inside thereof and containing a lipid compound of the formula (I) as a lipid component (R1 and R2 represent CH3—(CH2)n—CH═CH—CH2—CH═CH—(CH2)m—, n represents an integer of 3 to 5, m represents an integer of 6 to 10, p represents an integer of 2 to 7, and R3 and R4 represent a C1-4 alkyl group or a C2-4 alkenyl group.
Owner:HOKKAIDO UNIVERSITY
Ghrelin mimetic polypeptide hapten immunoconjugates having improved solubility and immunogenicity and methods of use thereof
ActiveUS9695222B2BioavailabilityImproving immunogenicityObesity gene productsDepsipeptidesPolyethylene glycolGrowth hormone-releasing peptide
Immunoconjugates for impeding weight gain and treating obesity in a subject are disclosed. The immunoconjugates comprise a ghrelin mimetic polypeptide hapten, a spacer moiety comprising one of more polyethylene glycol (PEG) units, and a protein carrier moiety. Immunoconjugates optionally include a conjugation moiety for conjugating the polypeptide hapten with a linker moiety or the protein carrier moiety and a linker moiety for conjugating the conjugation moiety with the protein carrier moiety.
Owner:THE SCRIPPS RES INST
Immune checkpoint inhibitor co-expression vectors
Disclosed herein are vectors that include antigen-encoding nucleic acid sequences and co-express immune modulators. Also disclosed are nucleotides, cells, and methods associated with the vectors including their use as vaccines.
Owner:GRITSTONE BIO INC
DNA VACCINE CONTAINING SPECIFIC EPITOPE OF APOLIPOPROTEIN (a)
ActiveUS20160303211A1Treating and preventing arteriosclerosisAvoid inductionApolipeptidesViral antigen ingredientsEpitopeHepatitis B virus core Antigen
The present invention provides an agent for the treatment or prophylaxis of arteriosclerosis comprising an expression vector encoding a chimeric Hepatitis B virus core antigen polypeptide inserted with an amino acid sequence containing a specific epitope of apolipoprotein (a), wherein the amino acid sequence containing the specific epitope is inserted between the amino acid residues 80 and 81 of the hepatitis B virus core antigen polypeptide.
Owner:ANGES
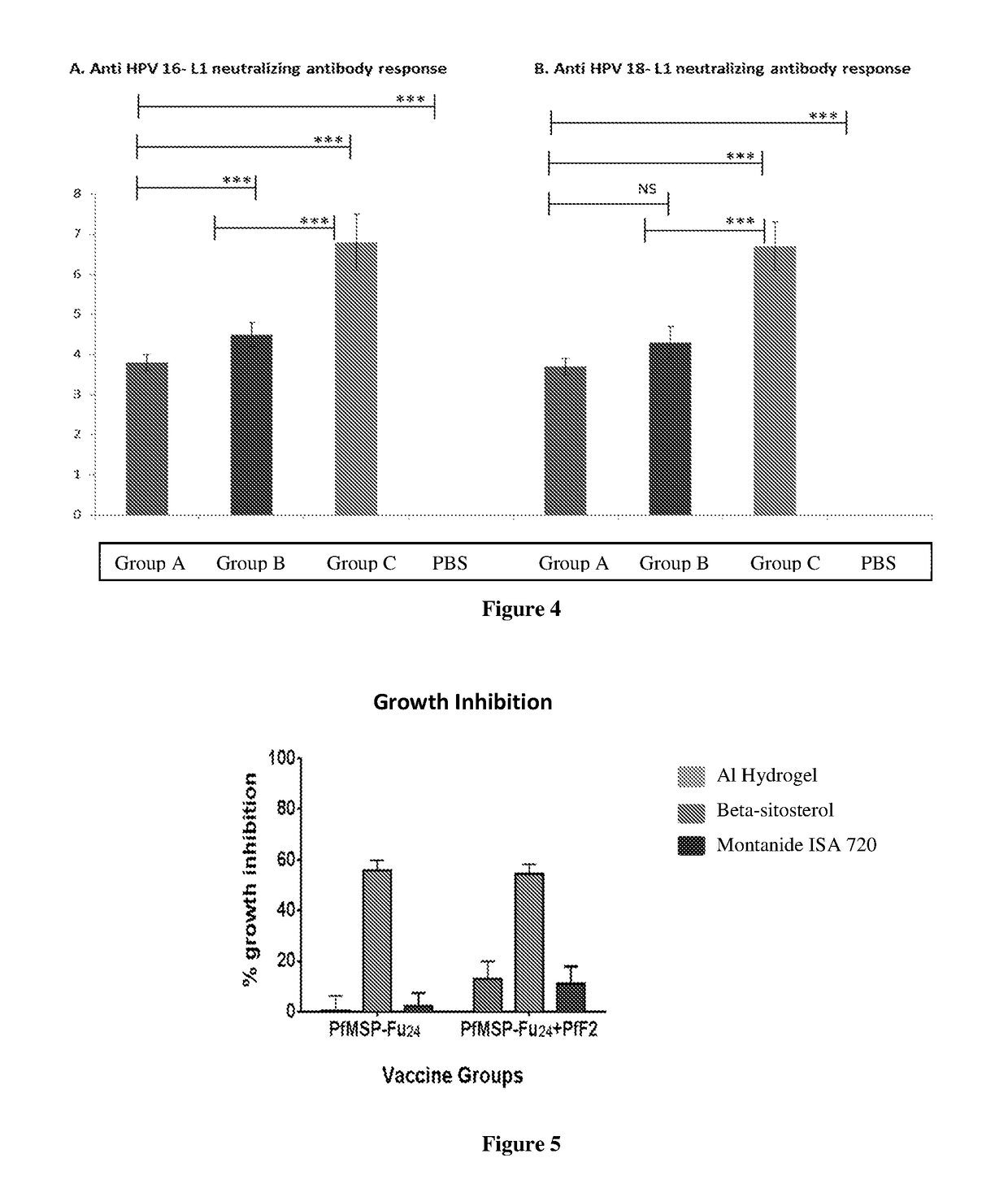
New adjuvant and vaccine composition containing the same
The present invention relates to certain polyphenol(s) having adjuvant property that can be used for the vaccine preparation. Also, the current invention provides adjuvant system comprising said polyphenol(s) and delivery system such as an immunostimulating reconstituted influenza virosomes (IRIVs). The present invention illustrates the said polyphenol(s) or an adjuvant system comprising of such polyphenol(s) and IRIVs can provide better level of immune response against antigen of interest than conventional vaccine systems. The preferred polyphenol according to the present invention can be beta-sitosterol. Beta-sitosterol can be optionally combined with the known adjuvant(s) to enhance immune response.
Owner:CADILA HEALTHCARE LTD
Methods for treating kidney disease with fragments of ApoB-100
The invention provides compositions comprising immunogenic fragments of ApoB-100 for eliciting an immune response in a subject or vaccinating a subject, so as to treat, prevent, 5 inhibit and / or reduce symptoms of kidney diseases in the subject. The compositions include immunogenic fragments of ApoB-100, CD8+ T cells activated with immunogenic fragments of ApoB-100 or a combination thereof.
Owner:CARDIOVAX
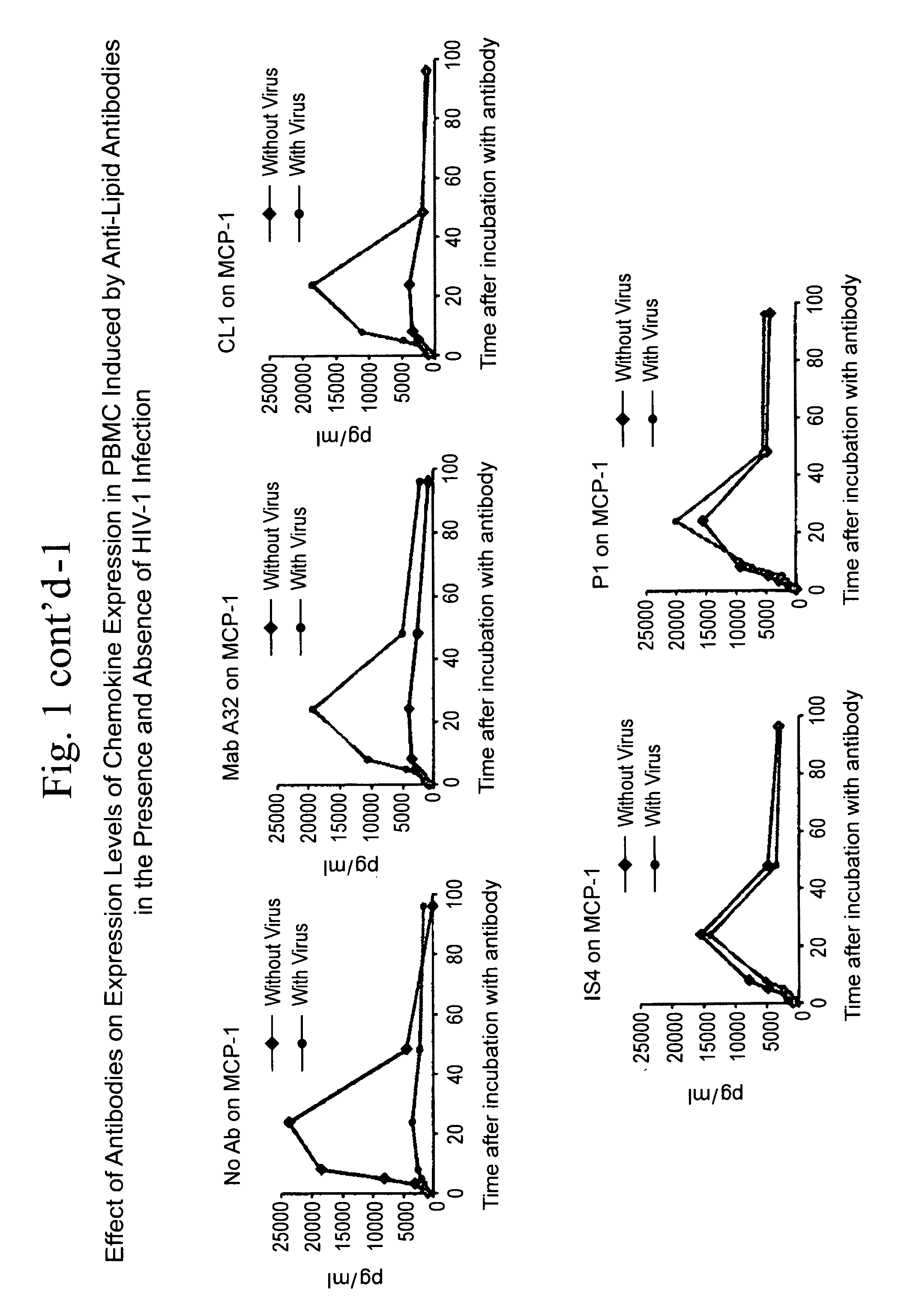
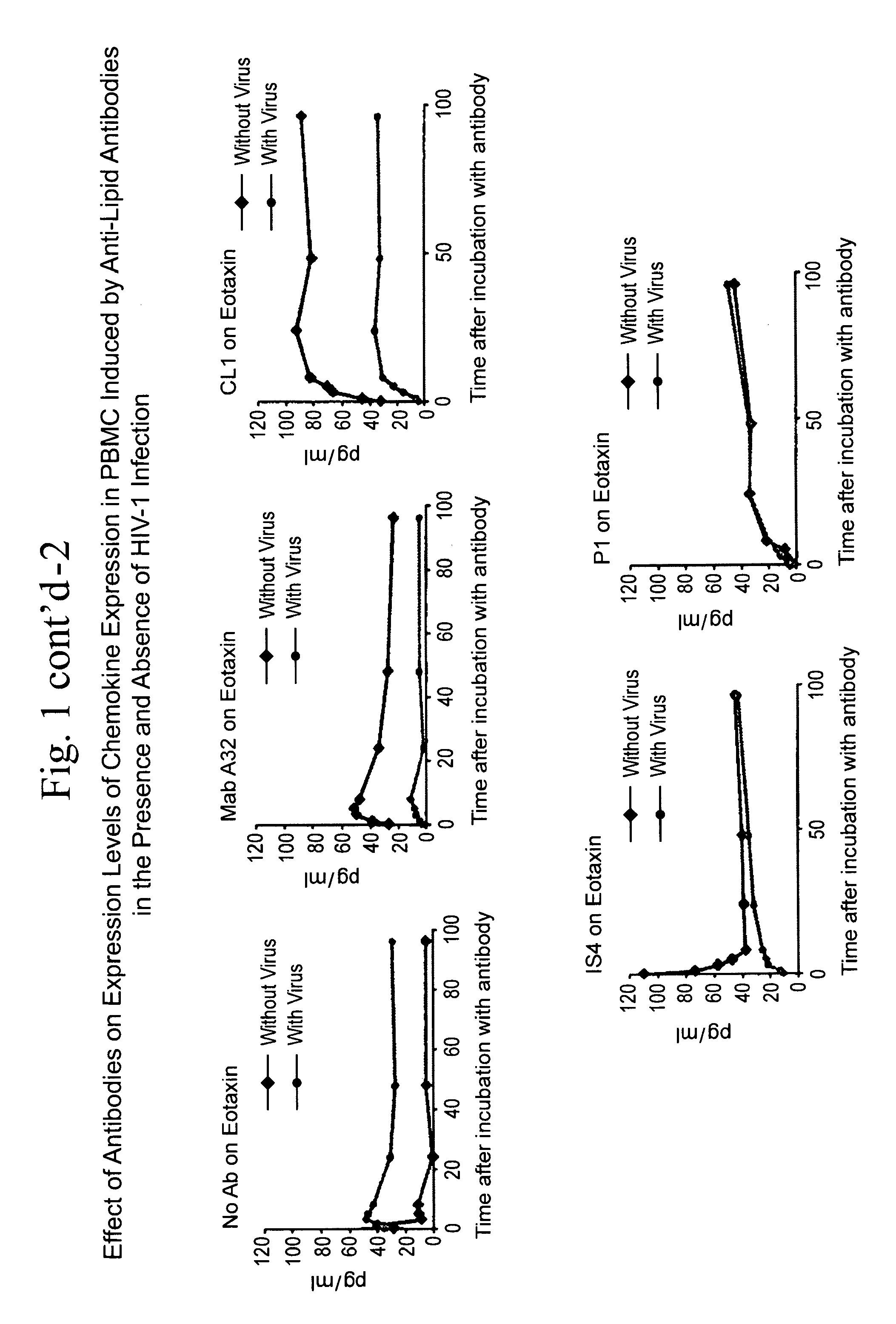
Method of inducing an Anti-viral immune response
InactiveUS20110262526A1Rapid anti-viral immune responseAvoid virus infectionOrganic active ingredientsAntiviralsAntigenAnti viral immunity
The present invention relates to a method of inducing an anti-viral immune response. The method comprises administering to a patient in need thereof an antigen that induces the production of antibodies that, upon binding to a cell surface target, result in the production of chemokines that inhibit viral infection.
Owner:DUKE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com