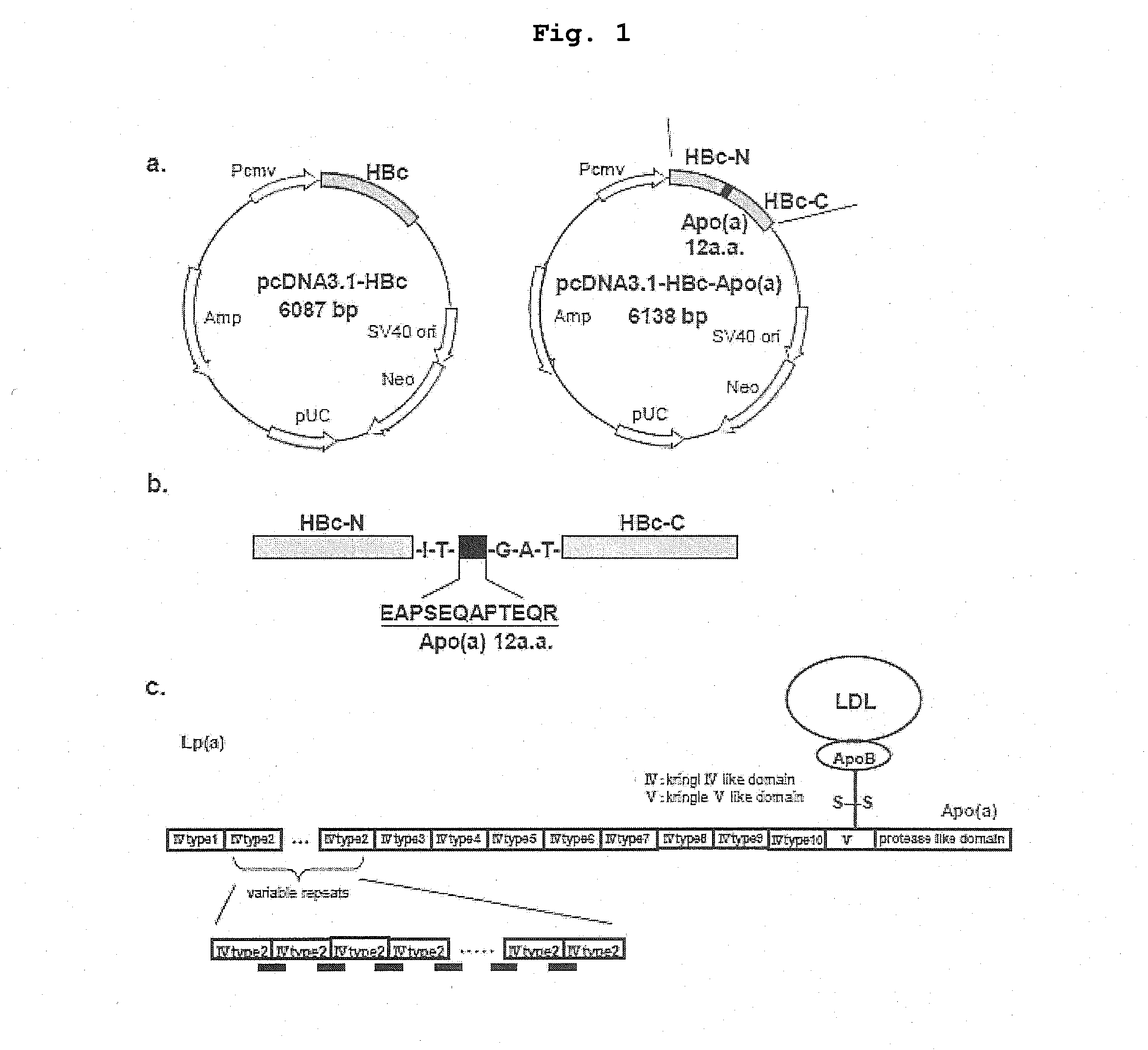
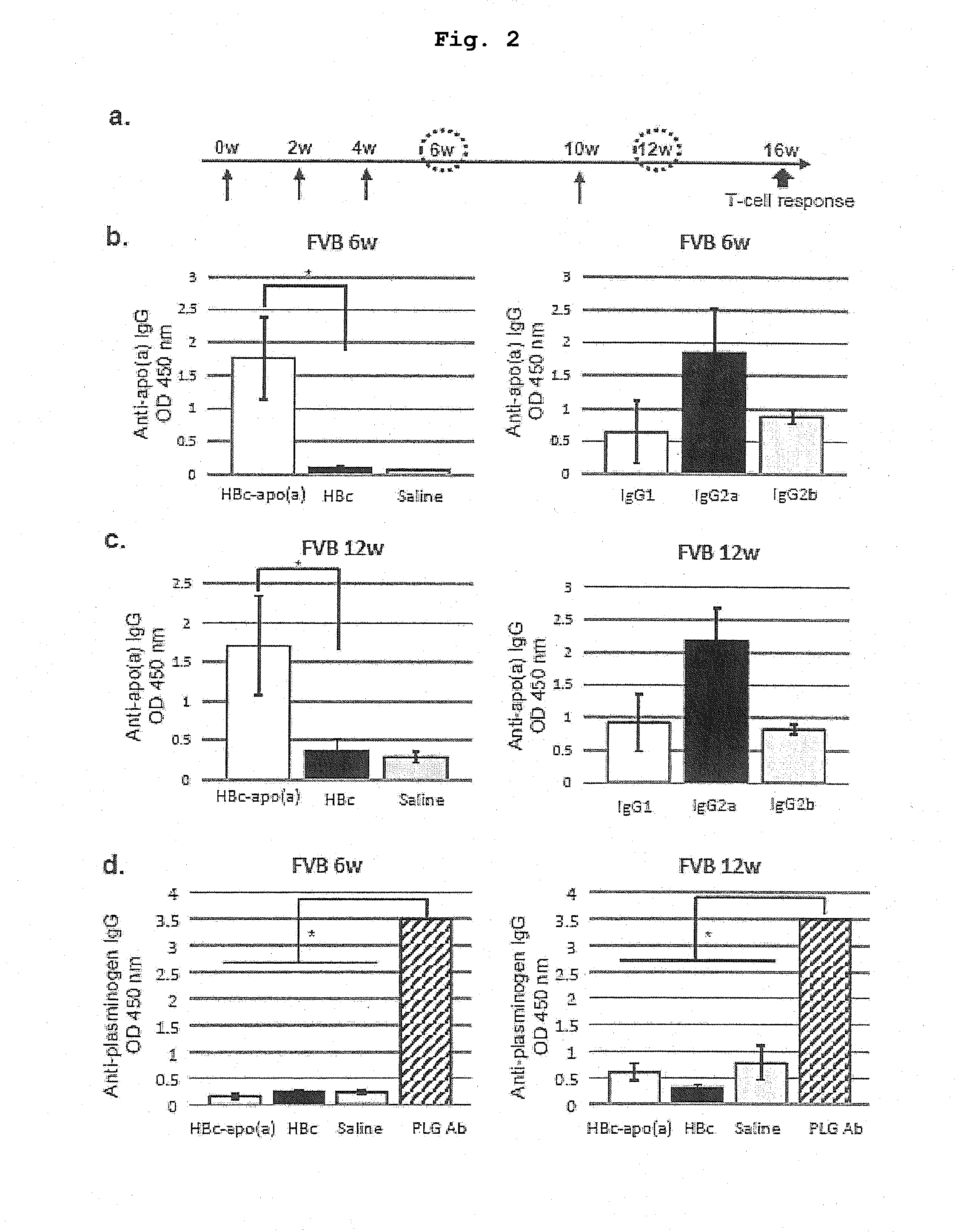
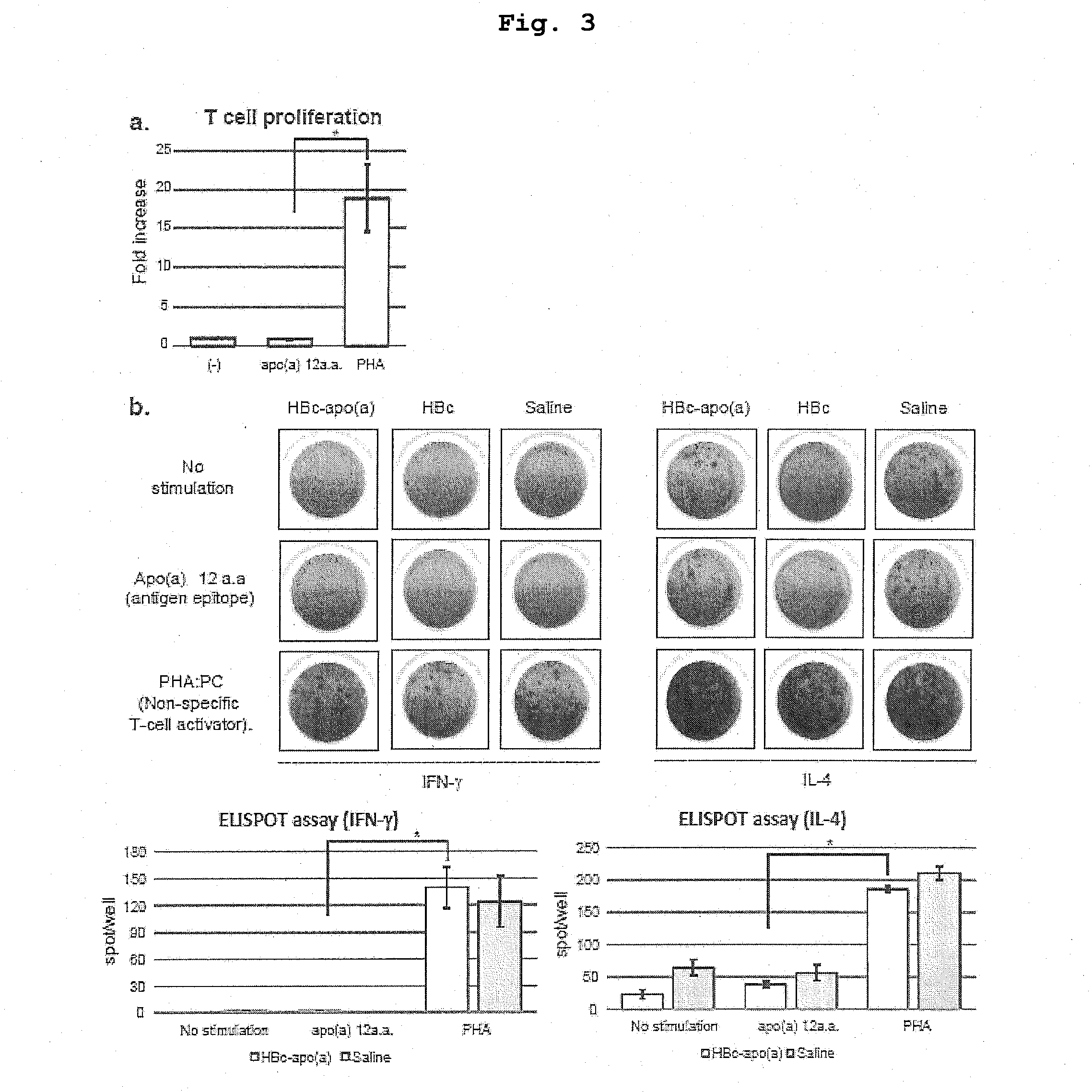
DNA VACCINE CONTAINING SPECIFIC EPITOPE OF APOLIPOPROTEIN (a)
a technology of apolipoprotein and dna vaccine, which is applied in the field of dna vaccine, can solve the problems of affecting the treatment effect of arteriosclerosis, affecting the effect of homeostatic clearance, and affecting the effect of anti-apolipoprotein activity, so as to reduce the risk of an adverse influence of self-reactive cellular immunity, prevent or treat arteriosclerosis, and avoid the induction of self-reactive t cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Animals
[0094]The experiments were approved by the Ethical Committee for animal experiments of Osaka University Graduate School of Medicine. Mice had free access to water and food during the experimental periods. Female FVB mice were purchased from Charles River. Lp(a) transgenic mice were created by the mating of human apo(a) transgenic mice and human apoB transgenic mice (Nature genetics, 1995, 9: 424-431; Nature, 1992, 360: 670-672; Proceedings of the National Academy of Sciences, 1994, 91: 2130; Circulation, 2002, 105: 1491-1496). Human apo(a) YAC transgenic mice were created by insertion of human apo(a) YAC transgenic mice, including the apo(a) gene, 70 kb apo(a)-like gene, and the 260 kb genomic DNA (YAC DNA) (Nature, 1992, 360: 670-672). Human apoB transgenic mice were created by insertion of 76 kb genomic DNA (P1 phagemid DNA) containing the intact apoB gene (Proceedings of the National Academy of Sciences, 1994, 91: 2130). The background of both mice was...
example 2
[0111]Carotid artery ligation model in Lp(a) transgenic mice in Example 1 was further analyzed.
[0112]In order to evaluate neutralization activity of the antibody induced by HBc-apo(a) vaccination, expression of inflammatory cytokines (IL-1β, TNF-α, MCP-1) induced by Lp(a) was analyzed using real-time PCR in macrophages differentiated from THP-1 cells in the presence of sera from immunized (apo(a) vaccination) or control group mice. As a result, serum from mice vaccinated by HBc-apo(a) significantly inhibit LP(a) induced IL-1β, TNF-α, and MCP-1 expression in macrophase as compared to control mice serum (FIG. 6). This result suggests that HBc-apo(a) vaccination induces neutralizing antibody against apo(a), and the neutralizing antibody binds to Lp(a) in a blood to suppress inflammatory cytokine production due to Lp(a) stimulation, decreases the amount of inflammatory cytokine in the blood, thereby suppressing intimal thickening (i.e. arteriosclerosis).
[0113]In addition, Lp(a) depositi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com