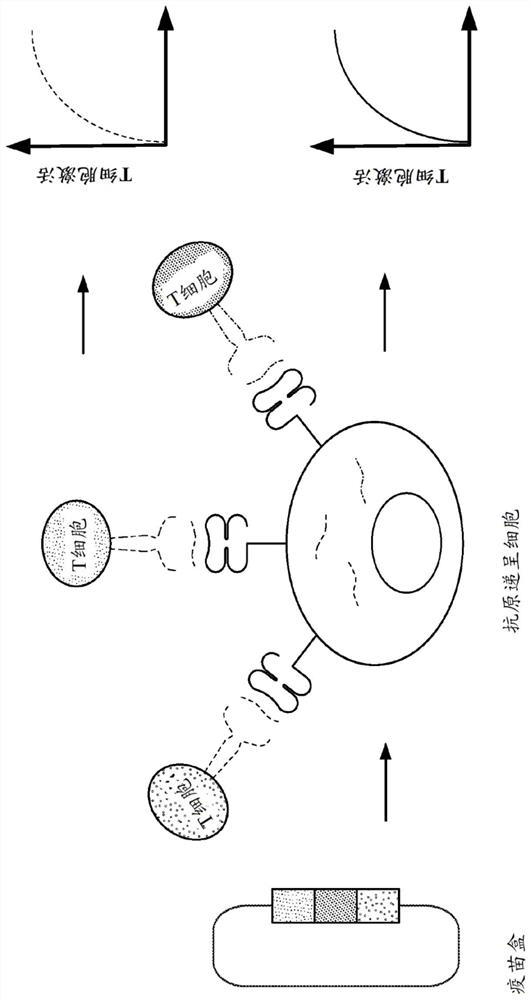
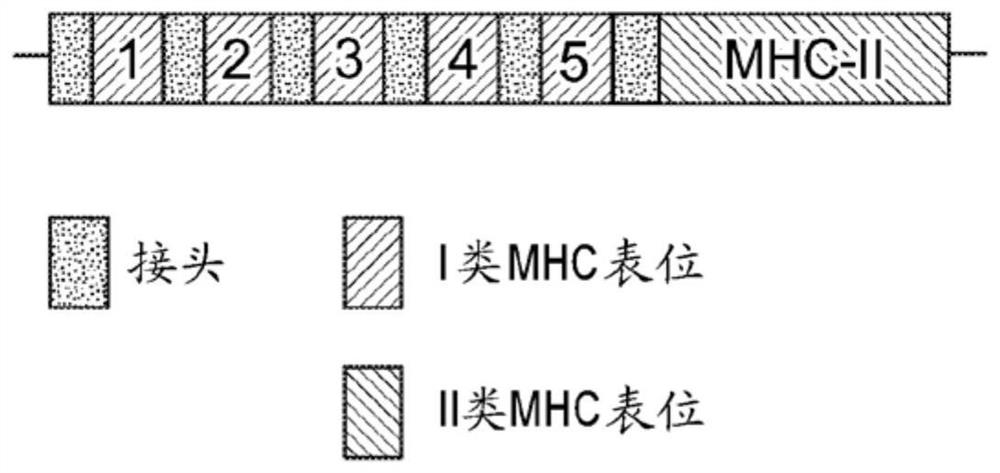
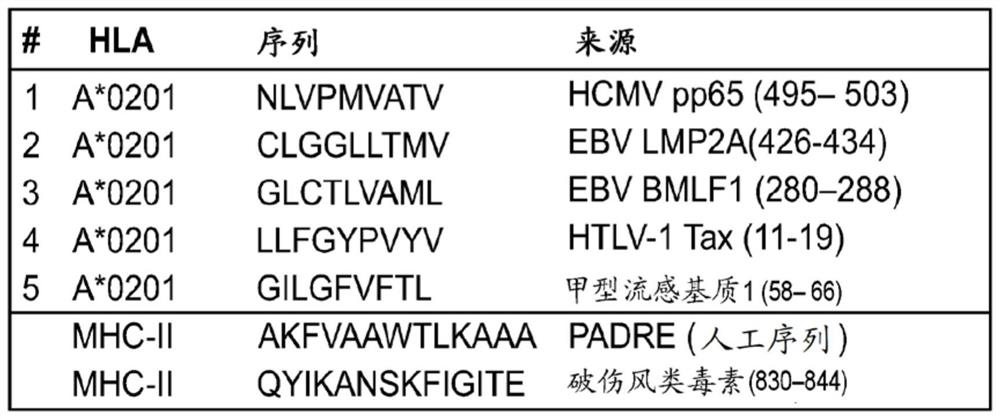
Immune checkpoint inhibitor co-expression vectors
A carrier system, immune regulation technology, applied in the direction of immunoglobulin, introduction of foreign genetic material using carrier, vaccine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0142] I. Definition
[0143] Generally, the terms used in the claims and specification are intended to be interpreted to have their ordinary meanings as understood by those of ordinary skill in the art. For clarity, certain terms are defined below. In the event of a conflict between the ordinary meaning and the provided definition, the provided definition shall apply.
[0144] As used herein, the term "antigen" is a substance that induces an immune response. An antigen can be a neoantigen. Antigens may be "consensus antigens," which are antigens found in a particular population (eg, a particular population of cancer patients).
[0145] As used herein, the term "neoantigen" is an antigen that has at least one alteration that makes it different from the corresponding wild-type antigen, for example via a mutation in a tumor cell or a post-translational modification specific for a tumor cell. Neoantigens can include polypeptide sequences or nucleotide sequences. Mutations ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com