Peptide-based immunization therapy for treatment of atherosclerosis
a technology of atherosclerosis and immunization therapy, which is applied in the field of new peptides, can solve the problems that the immunization treatment of atherosclerosis using intact oxidised ldl could have a detrimental effect, and not always the case, and achieve the effect of protecting atherosclerosis and strong cellular immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
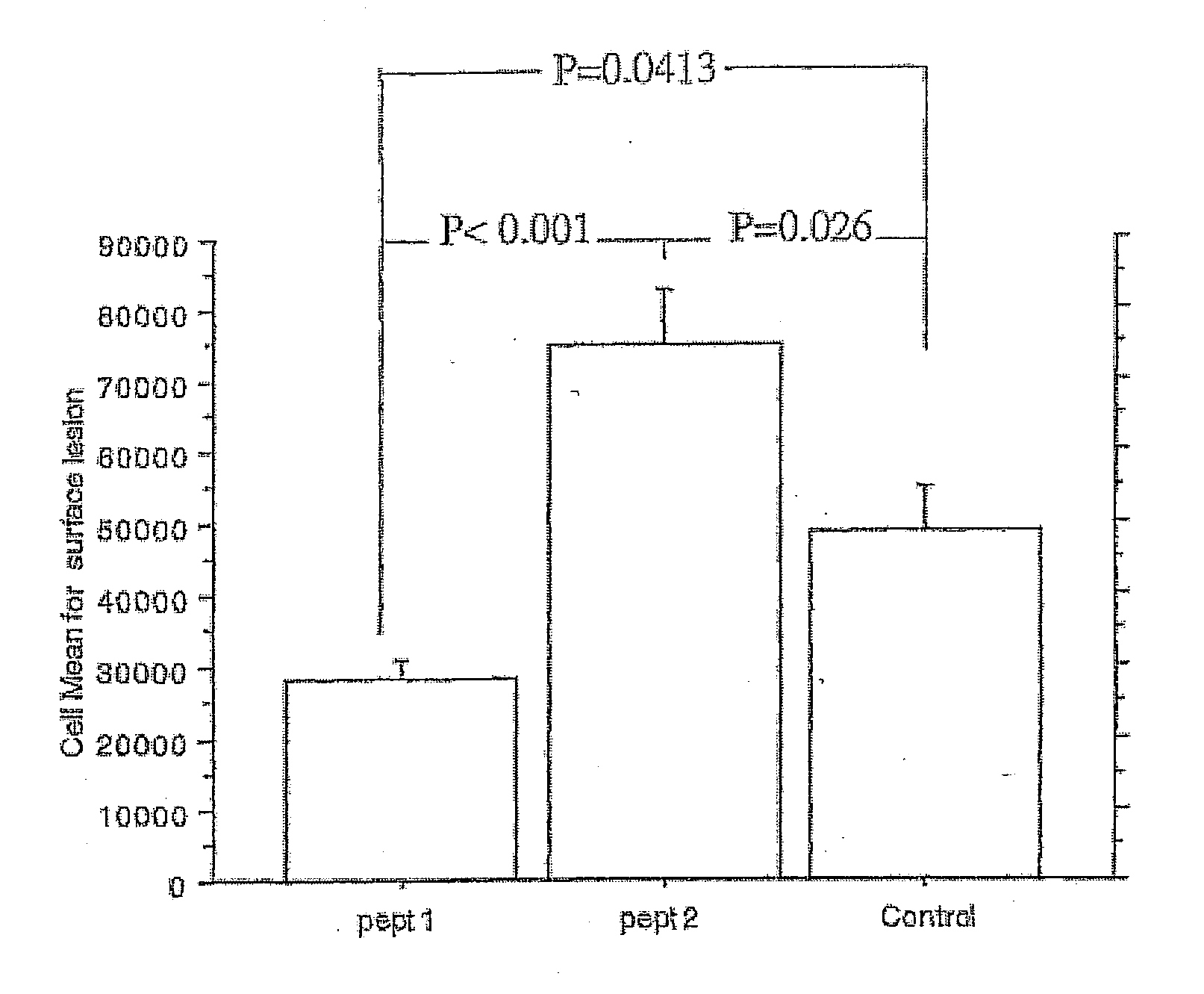
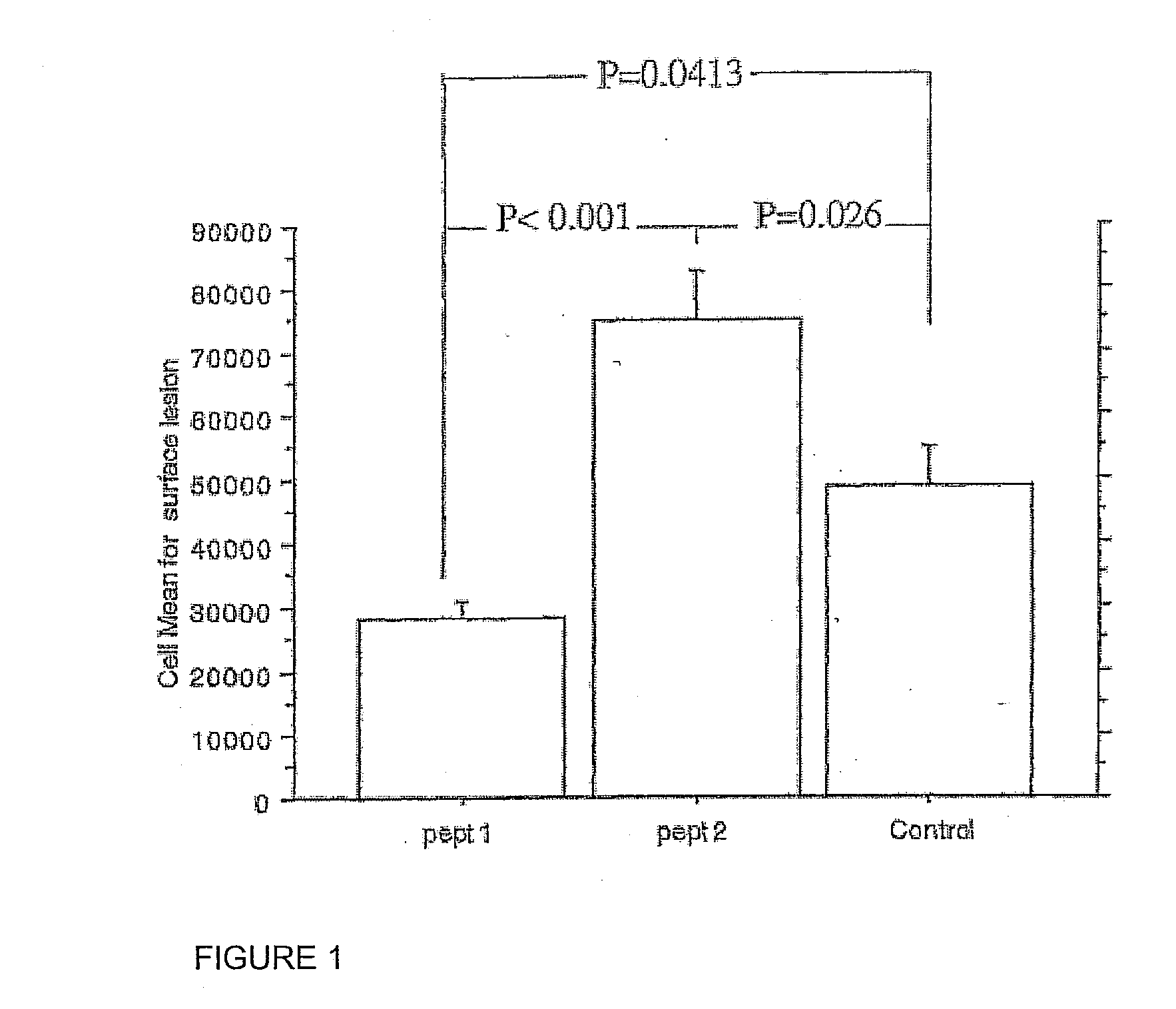
[0026] It has thereby been determined that the following peptide, when derivatized with MDA fulfills the criteria required to initiate a cellular immune response. This peptide is
NH2-PTVMDFRKFSRNYQLYKSVSLPS-COOHSEQ. ID. NO. 1
or an active part of this peptide.
[0027] The present peptide, SEQ. ID. NO 1, is based on amino acids 625-647 of the amino acid sequence of the human apoB100 protein.
[0028] When a peptide synthesized to contain this amino acid sequence is conjugated with MDA and injected into atherosclerosis-prone mice together with a suitable adjuvant, it reduces the development of atherosclerosis in the animals.
[0029] The present invention thus relates to a fragment of apoB intended for immunization or therapeutic treatment of mammals, including humans, against ischemic cardiovascular diseases and having immunological prophylactic or therapeutic properties against ischemic cardiovascular diseases, and / or diagnosing the presence or absence of antibodies related to increased...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| external radioactivity analysis | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com