Preparation method of 1Beta-methyl carbapenem antibiotic bicyclic mother nucleus
A technology of methyl carbapenem and antibiotics, which is applied in the field of preparation of 1β-methyl carbapenem antibiotic bicyclic nucleus, can solve the problems of high product destructiveness, severe reaction exotherm, difficult separation of products, etc., and achieves The effect of reducing solvents, high quality, and eliminating potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
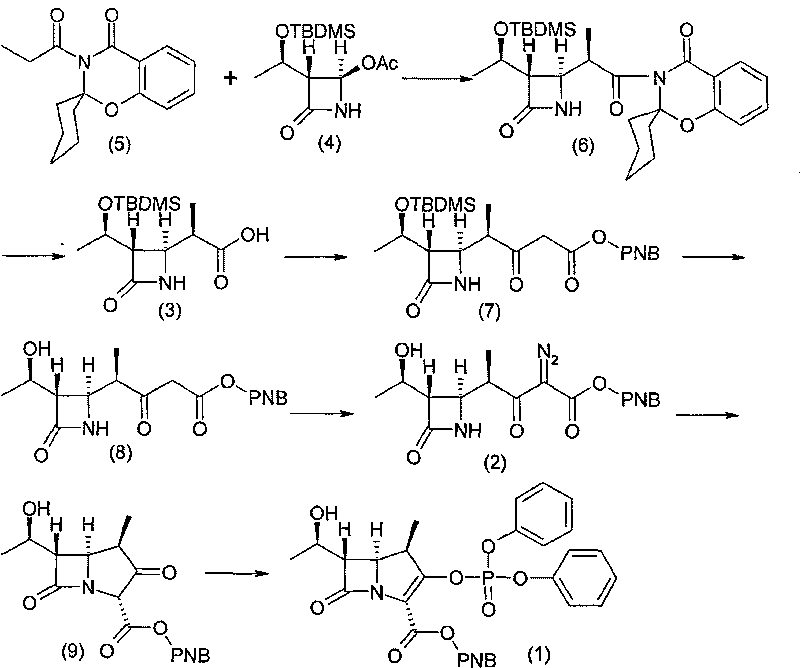
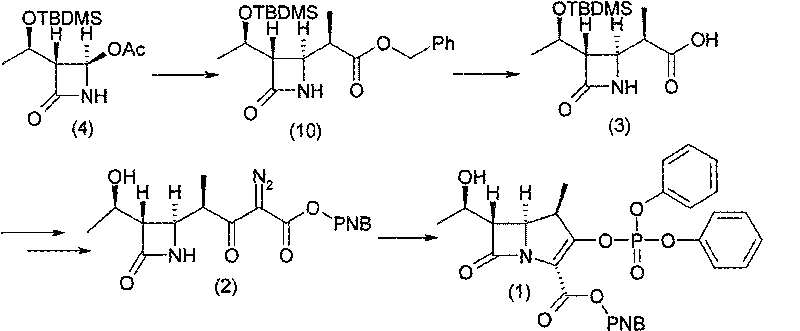
[0053] Example 13-{(2R)-2-[(3S,4R)-3-[(1R)-1-(tert-butyldimethylsilyloxy)ethyl]azetidin-2-one-4 Synthesis of -yl]propionyl}spiro-[2H-1,3-benzoxazin-2,1`-cyclohexane]-4-(3H)-one compound (6)
[0054] Under the protection of nitrogen, add 400ml of dichloromethane and 85.5g of compound (5) into a 1000ml four-necked reaction flask, stir to dissolve, cool the mixture to -10°C, control the temperature at -10~-5°C, and add 62.5g of Titanium tetrachloride, drop in 40 minutes, then add dropwise alkali mixed solution (35g triethylamine and 0.6g 4-dimethylaminopyridine diluted with 20ml dichloromethane) at the same temperature, drop in 40 minutes, heat preservation reaction After 2 hours, add 60g of compound (4), keep the reaction at -5~0°C for 30 minutes, then raise the temperature to 20~25°C and keep it for 5 hours. Sodium aqueous solution and water were washed, and the solvent was concentrated under reduced pressure to obtain a concentrated residue containing compound (6).
Embodiment 2
[0055] Example 2 (3S, 4R)-3-[(1R)-1-(tert-butyldimethylsiloxy)ethyl]-4-[(1R)-1-methyl-1-carboxyethyl] -Synthesis of azetidin-2-one compound (3)
[0056] Add 600ml of acetone to the residue containing compound (6) obtained in step 1), stir to dissolve, then add 60ml of water, cool down to -5~0°C, add 135g of 30% hydrogen peroxide, and add in batches at -5~0°C Add 25.8g of lithium hydroxide monohydrate within 30 minutes, keep warm at 0-5°C for 4 hours, TLC detects that the reaction of the raw materials is complete, add 15% (w / v) sodium sulfite solution dropwise in an ice-salt bath to neutralize excess hydrogen peroxide, Filter, rinse the filter cake with 200ml of water, add 300ml of dichloromethane to the filtrate to extract impurities, add 600ml of ethyl acetate to the water layer, adjust the pH value to 1~2 with 4mol / L hydrochloric acid solution, separate layers, and use the ethyl acetate layer Wash with saturated brine, add anhydrous sodium sulfate and activated carbon for d...
Embodiment 3
[0057] Example 3 (3S, 4R)-4-[(1R)-1-(tert-butyldimethylsilyloxy)ethyl]-3-[(1R)-1-methyl-3-(p-nitro Synthesis of Benzyloxycarbonyl)-2-oxopropyl]2-azetidin-2-one (7)
[0058] Under the protection of nitrogen, put 600ml of acetonitrile and 60g of compound (3) into a 1000ml four-necked reaction flask, add 42g of N,N'-carbonyldiimidazole in batches at a temperature of 20-25°C, and keep the reaction at 20-25°C for 30 minutes. Add 95g of p-nitromonobenzyl malonate and 30.5g of anhydrous magnesium chloride, add 60g of triethylamine dropwise at a temperature of 20-25°C, drop it within 1 hour, keep the reaction at 20-30°C for 10 hours, and cool to 10 Below ℃, add 600ml of ethyl acetate and 300ml of 3mol / L hydrochloric acid solution successively, stir until the reaction liquid is clear, let stand to separate layers, extract the product with ethyl acetate, use 5% (w / v) brine, 5% (w / v) successively v) Washing with potassium carbonate and 5% (w / v) brine, and ethyl acetate as the reaction s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com