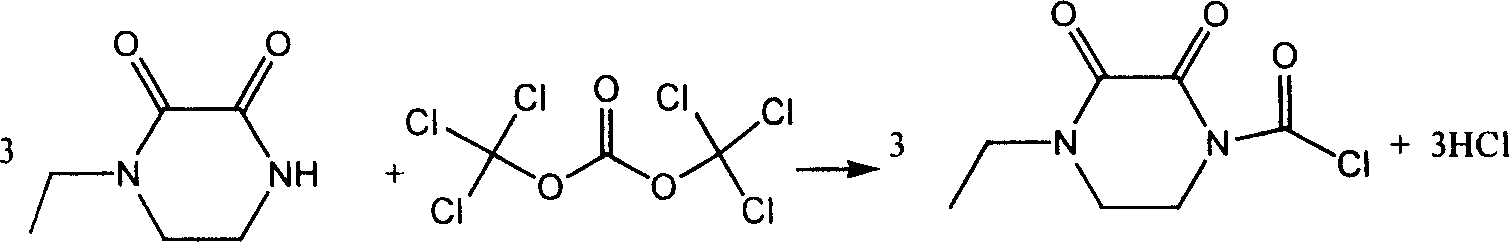
Chemosynthesis method of 1-chloroformyl-4-ethyl-2, 3-dioxopiperazine
A technology of dioxopiperazine and chloroformyl, which is applied in the field of chemical synthesis of 1-chloroformyl-4-ethyl-2,3-dioxopiperazine, which can solve transportation and storage difficulties and potential safety hazards Large scale, difficult industrialization and other problems, to achieve the effect of high implementation value and social and economic benefits, safe and reliable production, and reasonable process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] The molar ratio of feeding is N-ethyl-2,3-dioxopiperazine:bis(trichloromethyl)carbonate:pyridine=1:0.35:1
[0012] Add N-ethyl-2,3-dioxopiperazine, dichloromethane and pyridine into the reaction kettle, and after stirring evenly, add dichlorobis(trichloromethyl)carbonate dropwise at 5°C within 60 minutes. Methane solution, then react at 15°C for 10h, after the reaction is complete, filter to recover pyridine hydrochloride, concentrate the filtrate to recover the solvent, refine it with acetone, and dry it in vacuum. The yield is 86.8%, the melting point is 94.6-96.0°C, and the content (HPLC) is 99.1%.
Embodiment 2
[0014] The molar ratio of feeding is N-ethyl-2,3-dioxopiperazine:bis(trichloromethyl)carbonate:pyridine=1:0.55:1
[0015] Add N-ethyl-2,3-dioxopiperazine, dichloromethane and pyridine into the reaction kettle, and after stirring evenly, add dichlorobis(trichloromethyl)carbonate dropwise at 5°C within 60 minutes. Methane solution, then react at 10-15°C for 8 hours, after the reaction is complete, filter and recover pyridine hydrochloride, concentrate the filtrate to recover the solvent, refine it with acetone, and dry it in vacuum. The yield is 87.2%, the melting point is 95.2-96.0°C, and the content (HPLC) is 99.6 %.
Embodiment 3
[0017] The molar ratio of feeding is N-ethyl-2,3-dioxopiperazine:bis(trichloromethyl)carbonate:pyridine=1:0.8:1
[0018] Add N-ethyl-2,3-dioxopiperazine, dichloromethane and pyridine into the reaction kettle, and after stirring evenly, add dichlorobis(trichloromethyl)carbonate dropwise at 5°C within 60 minutes. Methane solution, then react at 15-20°C for 8 hours, after the reaction is complete, filter to recover pyridine hydrochloride, concentrate the filtrate to recover the solvent, refine it with acetone, and dry it in vacuum. The yield is 85.0%, the melting point is 94.0-96.1°C, and the content (HPLC) is 98.8%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com