Beta-lactamase inhibitor
A lactamase and inhibitor technology, applied in the field of β-lactamase inhibitors, can solve the problems of not enough to deal with the diversity of β-lactamase, and achieve the effect of strong inhibitory effect and long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
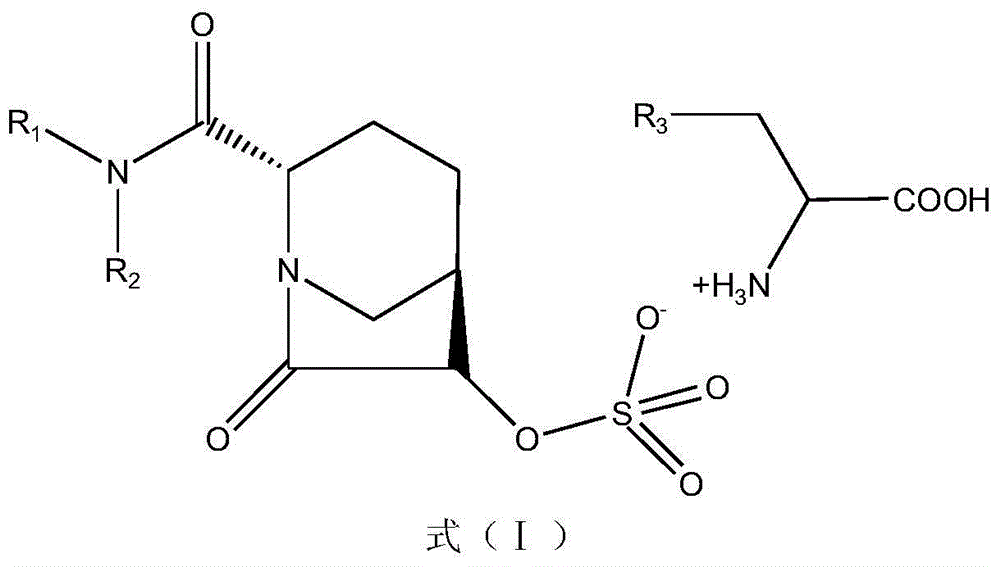
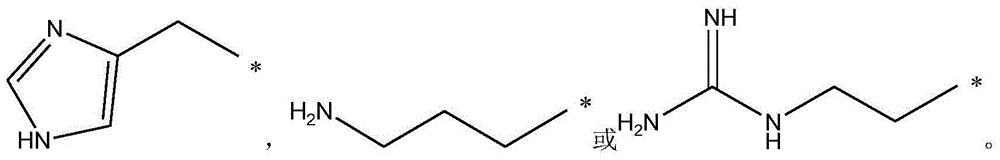
[0041] 1-1a compound (2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-ylsulfuric acid (S)-1-carboxy-2 -(1H-imidazol-4-yl)-ethylammonium, molecular formula C 13 H 20 N 6 O 8 S, molecular weight 420.11.
[0042] The preparation method is as follows:
[0043]
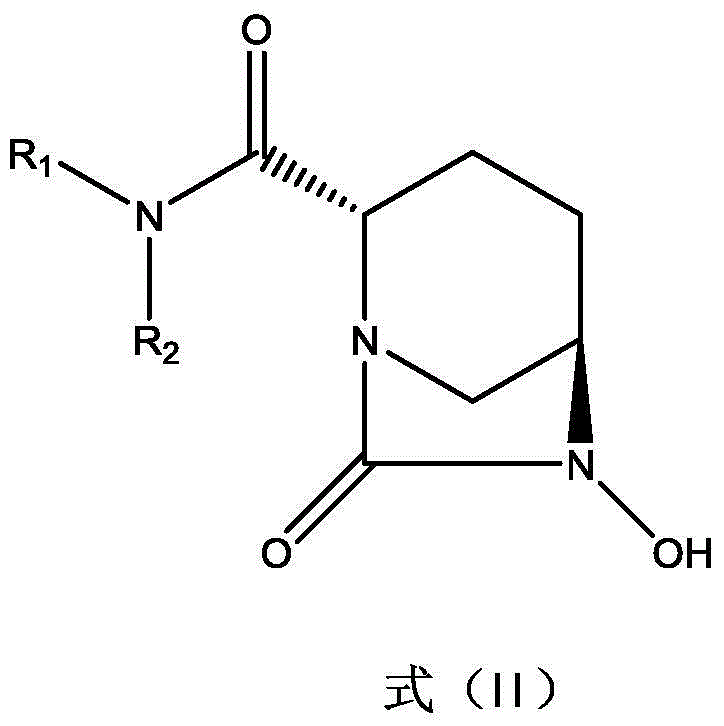
[0044] Dissolve 10.65g (50mmol) of (2S,5R)-6-hydroxy-7-oxo-1,6-diaza [3,2,1]octane-2-carboxamide in pyridine (35mL) Add triethylamine (2.5ml) and pyridine sulfur trioxide complex (9.6g), stir at room temperature for 12h, filter to remove the solid and wash with ethyl acetate, combine the filtrate and remove the solvent. The residue was dissolved in ethyl acetate (35ml), the insoluble matter was filtered off, a methanol (12ml) solution with 77.6g (50mmol) of histidine dissolved in it was added, the mixture was placed in an ice water bath at 0°C and kept stirring for 1h, filtered and used a small amount The precipitate was washed with ethyl acetate and dried to obtain 170.5 g of 1-1a white powder with a yield of 81%.
[00...
Embodiment 2
[0047] 1-1b(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-ylsulfuric acid (S)-1-carboxy-5- Amino-pentylammonium, molecular formula C 13 H 25 N 5 O 8 S, molecular weight 411.43.
[0048] The preparation method is as follows:
[0049]
[0050] Dissolve (2S,5R)-6-hydroxy-7-oxo-1,6-diaza[3,2,1]octane-2-carboxamide 92.6g (500mmol) in pyridine (350mL) Add triethylamine (25.0ml) and pyridine sulfur trioxide complex (95.5g), stir at room temperature for 12h, filter off the solid and wash with ethyl acetate, evaporate the solvent. The residue was dissolved in ethyl acetate (350ml), the insoluble matter was filtered off, and a solution of 73.1g (500mmol) of lysine dissolved in ethyl acetate (100ml) was added. The precipitate was washed with a small amount of ethyl acetate and dried to obtain 166.2 g of compound 1-1b as a white powder with a yield of 81%.
[0051] 1 HNMR (400MHz, DMSO-d6) δ: 6.11 (s, 2H), 4.85 (m, 1H), 4.55 (m, 1H), 3.79-3.68 (m, 1H), 3.34 (m, 2H), 3.06 (br ,2H),2.6...
Embodiment 3
[0053] 1-1c(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-ylsulfuric acid (S)-1-carboxy-4- Guanidine-Butylammonium, Molecular Formula C 13 H 25 N 7 O 8 S, molecular weight 439.44.
[0054] The preparation method is as follows:
[0055]
[0056] Dissolve (2S,5R)-6-hydroxy-7-oxo-1,6-diaza[3,2,1]octane-2-carboxamide 92.6g (500mmol) in pyridine (350mL) Add triethylamine (25.0ml) and pyridine sulfur trioxide complex (95.5g), stir at room temperature for 12h, filter off the solid and wash with ethyl acetate, evaporate the solvent. The residue was dissolved with ethyl acetate (350ml), the insoluble matter was filtered off, a solution of 87.1g (500mmol) of arginine hydrochloride dissolved in methanol (150ml) was added, and the mixture was placed in an ice water bath at 0°C and stirred for 1h The precipitate obtained by filtration was washed with a small amount of ethyl acetate and dried to obtain 171.1 g (78%) of white powder of compound 1-1c.
[0057] 1 HNMR (400MHz, DMSO-d6) δ: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com