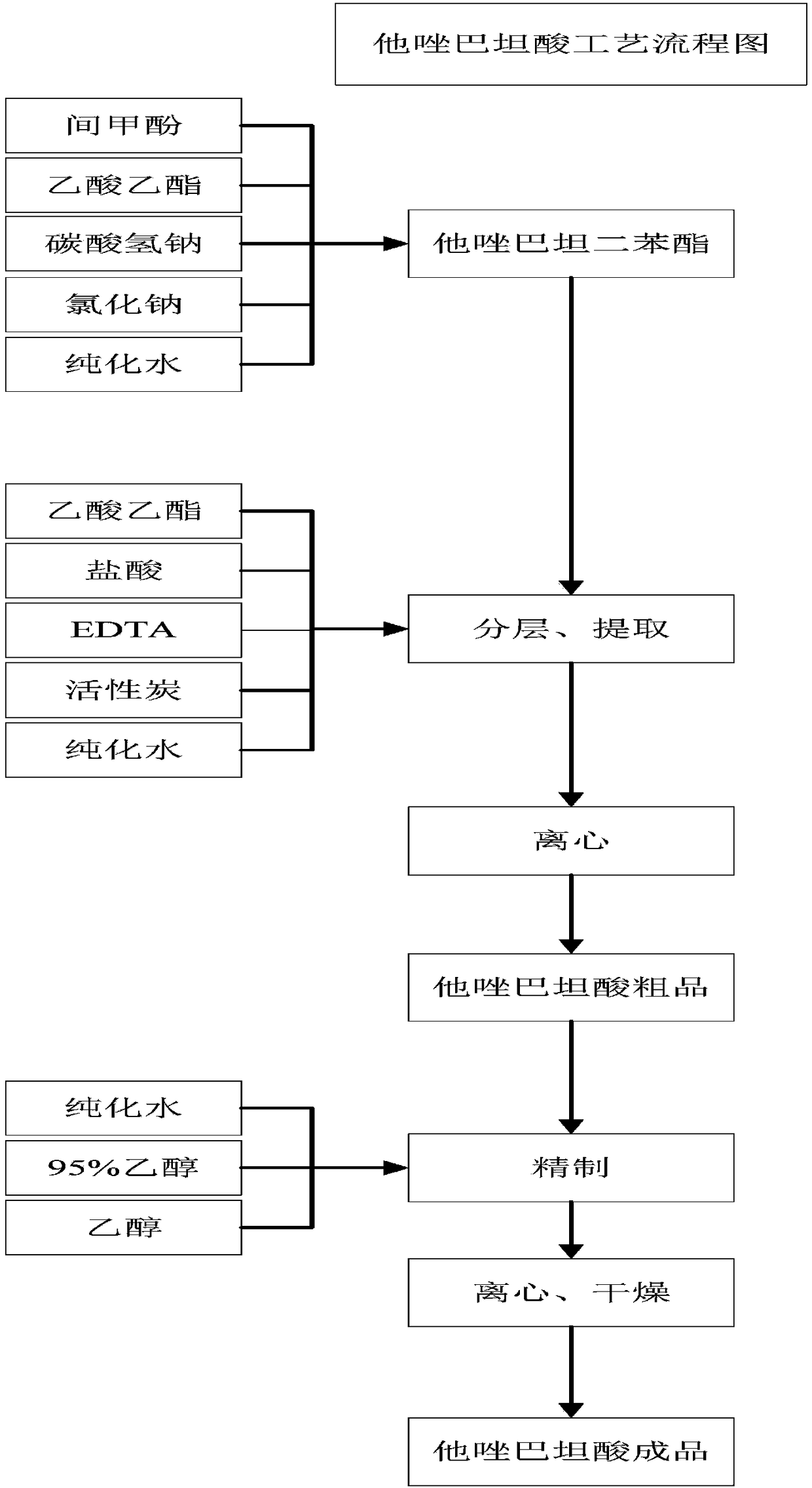
Preparation technologies of tazobactam acid and tazobactam diphenylmethyl ester and application
A technology of diphenylmethyl zobactam acid and tazobactam acid, which is applied in the field of organic polymer synthesis technology, can solve problems such as low yield, achieve the effects of reducing production costs, reducing toxicity and harm, and increasing sales profits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
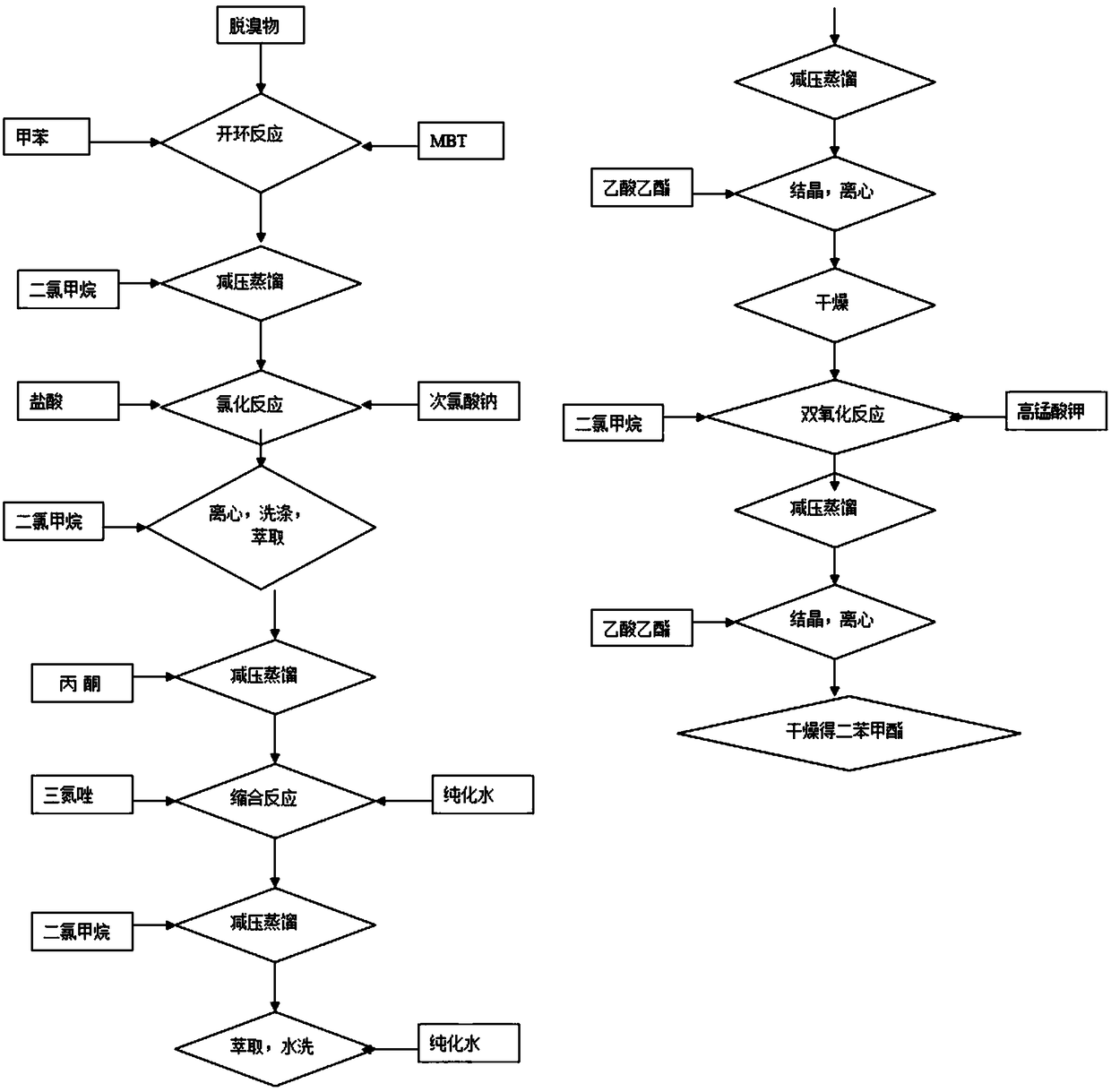
[0067] The preparation process flowchart of tazobactamic acid, such as figure 1 As shown, it mainly includes the following steps:
[0068] Step 1: ring-opening reaction;
[0069] Step 2: chlorination reaction;
[0070] Step 3: condensation reaction;
[0071] Step 4: double oxidation reaction.
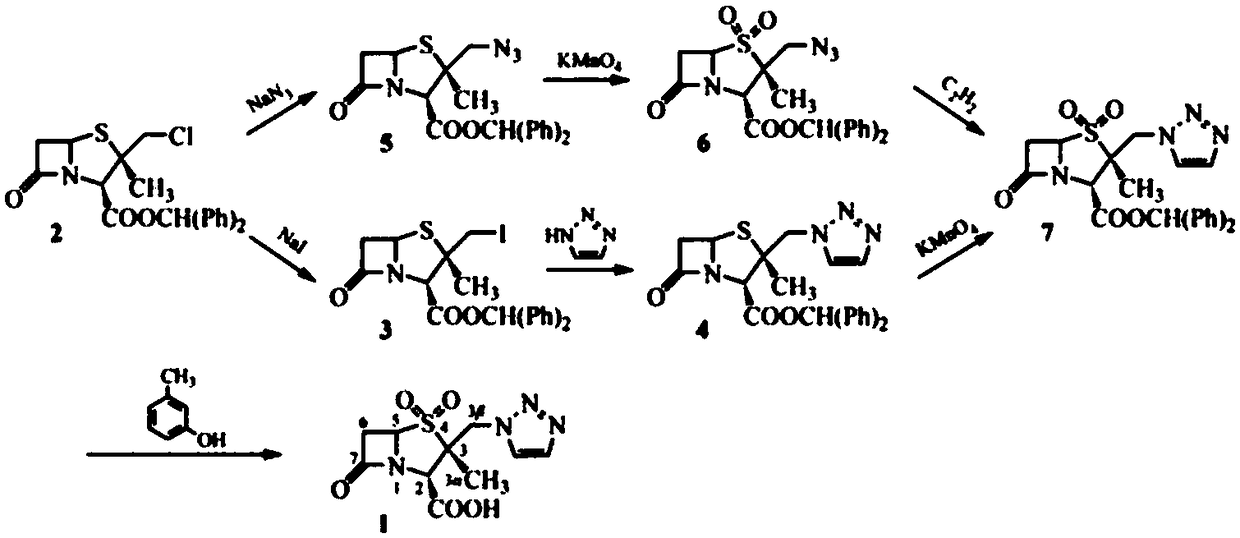
[0072] The reaction equation for the ring-opening reaction is as follows:
[0073]
[0074] The preparation steps are as follows:
[0075] Step 1.1: Put 3.0 parts of toluene, 0.2 parts of debrominated product and 0.1 part of MBT into reaction vessel A, heat up to 70°C and react for 80-100 minutes;
[0076] Step 1.2: After the reaction is completed, cool down to 58°C;
[0077] Step 1.3: Concentrate the product in step 1.2 to dryness under reduced pressure, add 2.3 parts of dichloromethane and stir until completely dissolved to obtain a clear liquid, and obtain a dichloromethane solution of the ring-opener.
[0078] The reaction equation of the chlorination reaction is as follow...
Embodiment 2
[0101] Step 1: Ring Opening Reaction
[0102] Step 1.1: Put 3.5 parts of toluene, 0.4 part of debrominated product and 0.2 part of MBT into reaction vessel A, heat up to 70-75°C and react for 80-100 minutes;
[0103] Step 1.2: After the reaction is completed, cool down to 75°C;
[0104] Step 1.3: Concentrate the product in step 1.2 to dryness under reduced pressure, add 2.5 parts of dichloromethane and stir until completely dissolved to obtain a clear liquid, and obtain a dichloromethane solution of the ring-opener;
[0105] Step 2: Chlorination reaction
[0106] Step 2.1: Transfer the dichloromethane solution of the ring-opening product in step 1.3 to the reaction vessel B, continuously stir and lower the temperature so that the dichloromethane solution of the ring-opening product in the reaction vessel B reaches 0-5°C;
[0107] Step 2.2: Slowly add 0.5 part of hydrochloric acid solution with a concentration of 31% to the reaction vessel B, and the dropping temperature is l...
Embodiment 3
[0123] Step 1: Ring Opening Reaction
[0124] Step 1.1: Put 3.2 parts of toluene, 0.25 parts of debrominated product and 0.14 parts of MBT into reaction vessel A, heat up to 70-75°C and react for 80-100 minutes;
[0125] Step 1.2: After the reaction is completed, cool down to 65°C;
[0126] Step 1.3: Concentrate the product in step 1.2 to dryness under reduced pressure, add 2.37 parts of dichloromethane and stir until completely dissolved to obtain a clear liquid, and obtain a dichloromethane solution of the ring-opener;
[0127] Step 2: Chlorination reaction
[0128] Step 2.1: Transfer the dichloromethane solution of the ring-opening product in step 1.3 to the reaction vessel B, continuously stir and lower the temperature so that the dichloromethane solution of the ring-opening product in the reaction vessel B reaches 0-5°C;
[0129] Step 2.2: Slowly add 0.37 parts of hydrochloric acid solution with a concentration of 31% to the reaction vessel B, and the dropping temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com