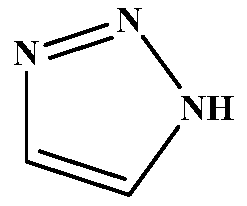
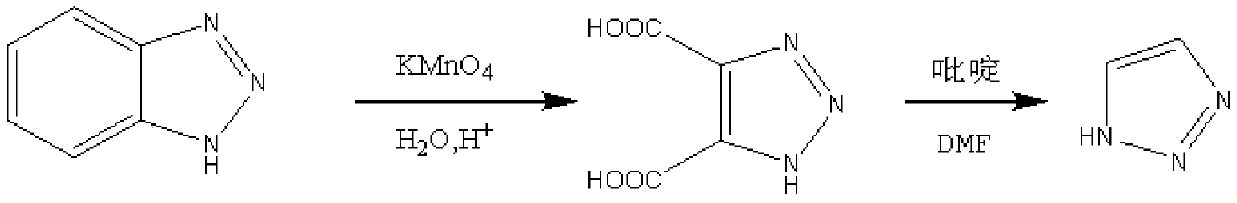
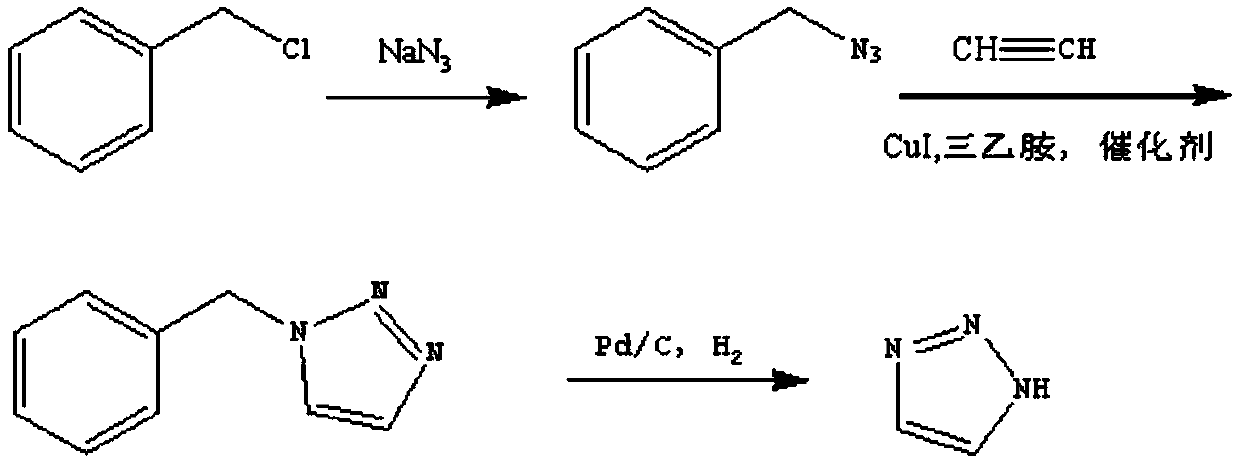
Method for preparing important intermediate 1H-1,2,3-triazole of Tazobactam
A technology of tazobactam and 1H-1, which is applied in the field of preparation of 1H-1,2,3-triazole, an important intermediate of tazobactam, can solve problems such as unsuitable scale-up production, harsh conditions and high price problems, to achieve broad market prospects and economic benefits, mild reaction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) In a 500ml three-neck flask, add 100ml methanol and 100g (0.69mol) 40% glyoxal solution, stir, and cool down to -5~5°C. 240g (1.72mol) of 50% hydroxylamine hydrochloride solution was added dropwise, and a white solid was gradually precipitated in the feed solution. After dropping, heat to reflux for 2h, and the solid dissolves. Cool down to -10-0°C for crystallization for 3 hours, filter, rinse the filter cake with 20ml of cold methanol to obtain a white wet product, and vacuum-dry at 30-40°C for 5 hours. 53.9 g of a white crystalline solid was obtained, namely Intermediate 1, with a purity of 98.72% and a yield of 88.9%.
[0049] (2) In a 1000ml three-neck flask, add 300ml of methanol, 51g (0.58mol) of intermediate one, 44.2g (0.70mol) of ammonium chloride, control the temperature at 25-35°C, stir, and dissolve the solid, slowly add 110g ( 0.70mol) 35% peracetic acid, the reaction solution gradually turned dark red, after the addition was completed, the heat pres...
Embodiment 2
[0054] (1) In a 1000ml three-neck flask, add 300ml of ethanol and 200g (1.38mol) of 40% glyoxal solution, stir, and cool down to -5~5°C. 425g (3.04mol) of 50% hydroxylamine hydrochloride solution was added dropwise, and a white solid was gradually precipitated in the feed solution. After dropping, heat to reflux for 2h, and the solid dissolves. Cool down to -10-0°C for crystallization for 3 hours, filter, rinse the filter cake with 50ml of cold ethanol to obtain a white wet product, and vacuum-dry at 30-40°C for 5 hours. 105.8 g of a white crystalline solid was obtained, namely Intermediate 1, with a purity of 98.55% and a yield of 87.2%.
[0055] (2) In a 1000ml three-necked flask, add 50% 300ml acetonitrile aqueous solution, 102g (1.16mol) of intermediate one, 174g (1.32mol) of ammonium sulfate, control the temperature at 25-35°C, stir, the solid dissolves, slowly add 86.8 g (1.28mol) 50% hydrogen peroxide, the reaction solution gradually turned dark red, after the additio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com