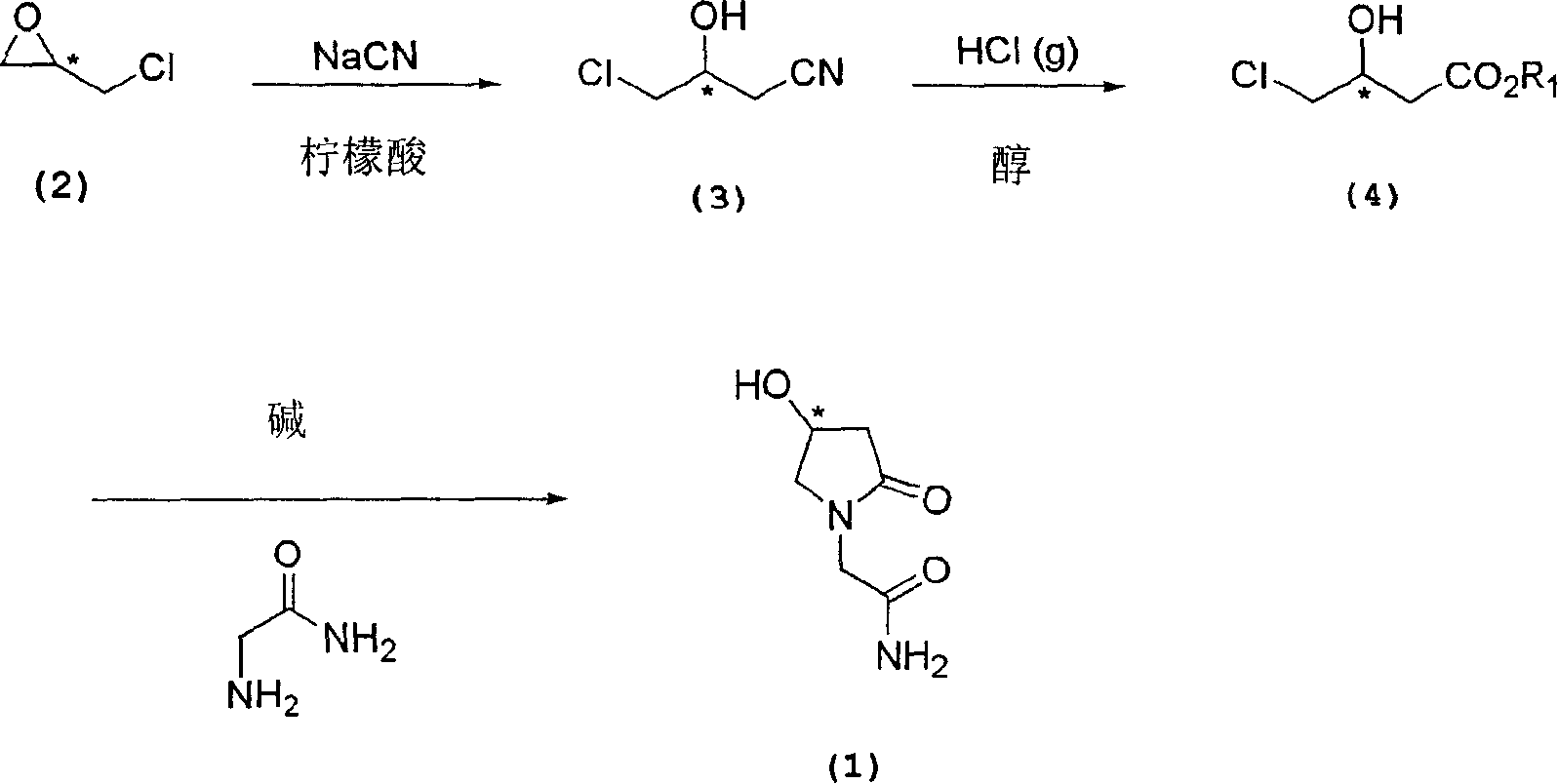
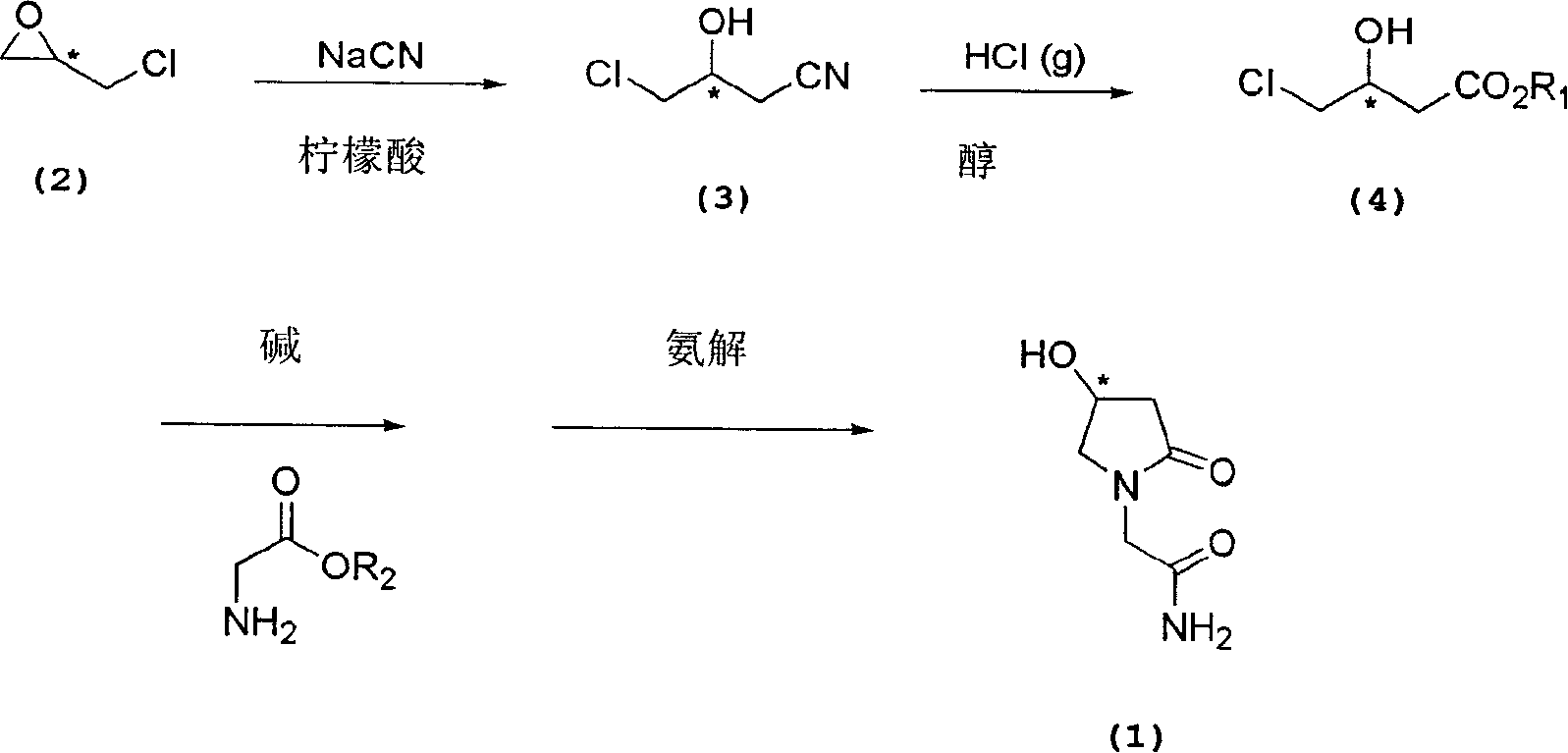
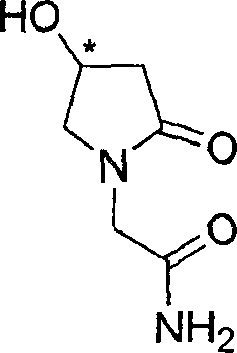
Process for the preparation of optically pure 4-hydroxy-2-oxo-1-pyrrolidine acetamide
A technology of pyrrolidineacetamide and hydroxyl, which is applied in the field of preparation of optically pure 4-hydroxy-2-oxo-1-pyrrolidineacetamide, can solve the problems of expensive raw materials, many steps, high cost, etc., and achieve simple procedures , the effect of increasing the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of (R)-3-chloro-2-hydroxy propionitrile
[0052] Into a 5 L 3-necked round bottom flask equipped with a thermometer, a pH meter and a stirrer, 400 g of water and 400 g of (R)-epichlorohydrin were sequentially added. 275 g of sodium cyanide dissolved in 347 g of water and 427 g of citric acid dissolved in 347 g of water were simultaneously added dropwise to the stirred solution. The pH and temperature of the reaction solution were maintained at 7.8-8.3 and 25°C-8.3°C, respectively. After the dropwise addition was completed, the temperature was raised to room temperature and stirred for another 10 hours. The reaction mixture was extracted with 2L (x2) ethyl acetate, and the organic layer was collected and dried over anhydrous magnesium sulfate. After filtration, the filtrate was evaporated under reduced pressure to obtain the target product chiral 3-chloro-2-hydroxypropionitrile.
Embodiment 2
[0053] Embodiment 2: the preparation of (S)-3-chloro-2-hydroxy propionitrile
[0054] Into a 5 L 3-necked round bottom flask equipped with a thermometer, a pH meter and a stirrer, 400 g of water and 400 g of (S)-epichlorohydrin were sequentially added. 275 g of sodium cyanide dissolved in 347 g of water and 427 g of citric acid dissolved in 347 g of water were simultaneously added dropwise to the stirred solution. The pH and temperature of the reaction solution were maintained at 7.8-8.3 and 25°C-8.3°C, respectively. After the dropwise addition was completed, the temperature was raised to room temperature and stirred for another 10 hours. 200 g of brine was added to the reaction mixture. The reaction mixture was partitioned in 5 L of ethyl acetate, and the ethyl acetate layer was separated. To the ethyl acetate solution was added 50 g of anhydrous sodium sulfate and stirred for 30 minutes. After filtration, the filtrate was evaporated under reduced pressure. The concentra...
Embodiment 3
[0056] Embodiment 3: the preparation of methyl-(S)-4-chloro-3-hydroxybutyric acid
[0057] Into a 3 L 3-necked round bottom flask equipped with a thermometer, a pH meter and a stirrer, 439 g of methanol were sequentially added and the temperature was lowered to -20°C. 372g of hydrogen chloride gas was supplied to the solution, and the temperature was maintained at -5°C to 0°C, and 458g of (S)-3-chloro-2-hydroxypropionitrile was added dropwise. After the dropwise addition was completed, the reaction temperature was raised to 20°C-25°C and stirred for 12 hours. The reaction mixture was evaporated under reduced pressure to remove methanol. To the residue was added 664 g of water and stirred for 1 hour. The aqueous solution was then extracted with 1.5 L (x2) of ethyl acetate, and the organic layer was collected and dried over anhydrous magnesium sulfate. After filtration, the filtrate was evaporated under reduced pressure. The residue was fractionally distilled to obtain 342 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com