Method for synthesizing 2-thiophene ethylamine
A synthetic method, the technology of thienylethylamine, applied in the field of medicine and chemical industry, can solve the problems of non-reusable, limited industrial application, expensive catalyst, etc., achieves low pollution, avoids the use of expensive or highly toxic substances, and has mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
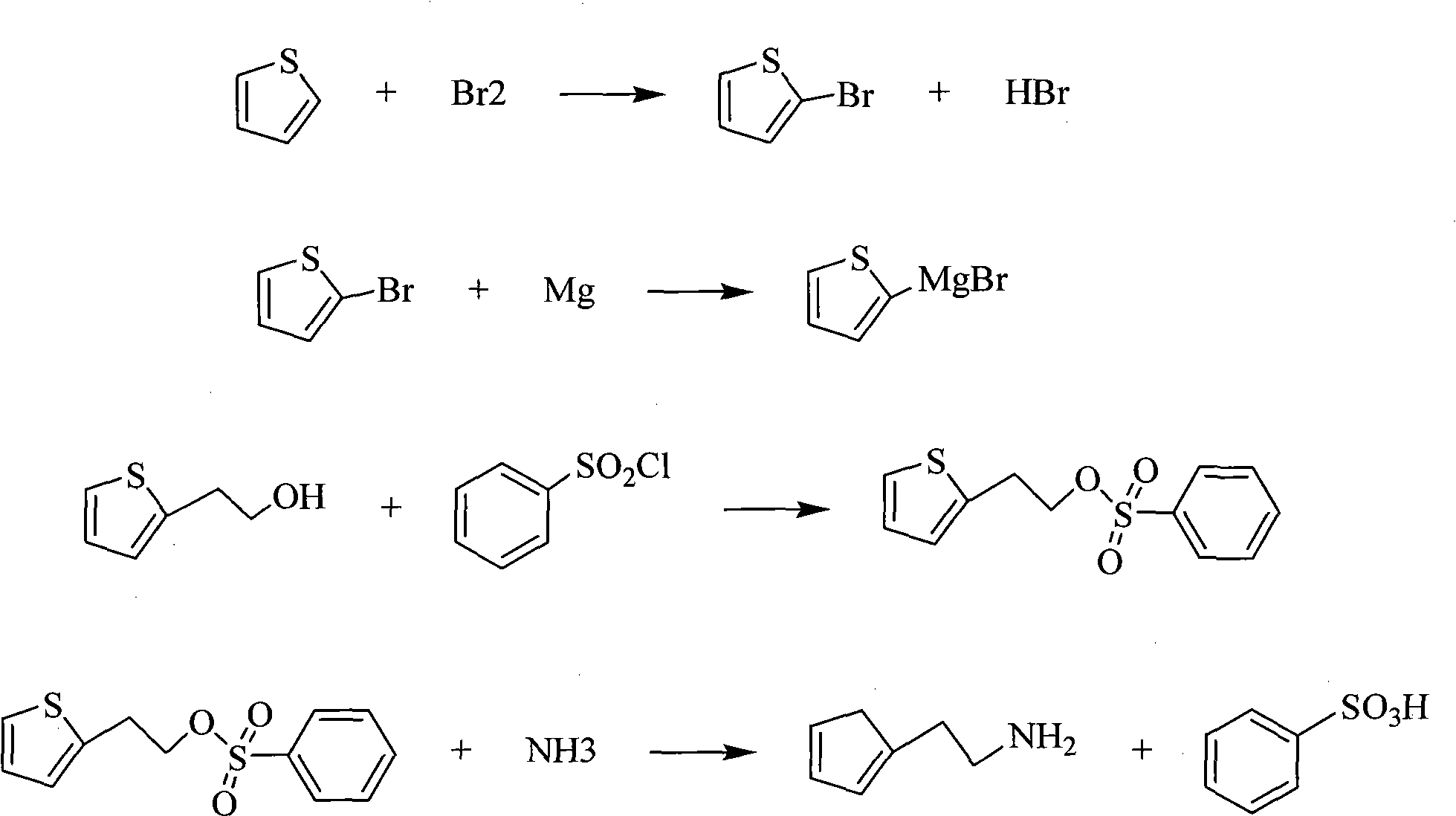
[0037] Example 1. A kind of synthetic method of 2-thienylethylamine, its step is as follows:
[0038] (1) Preparation of 2-bromothiophene: In the presence of an organic solvent, add a brominating agent dropwise to thiophene, control the reaction temperature at -10°C, keep the temperature for 2 hours after the dropwise addition, separate layers, remove the solvent, and then decompress The product is collected by distillation at 45°C / 1.73Kpa; the organic solvent is glacial acetic acid or carbon tetrachloride; the brominating agent is N-succinimide, pyridine hydrobromide, bromine or hydrogen bromide One of the acids; the molar ratio of the substance is: thiophene:brominating agent=1:2;
[0039] (2) Preparation of 2-thiophene ethanol: take 2-bromothiophene and magnesium chips to undergo Grignard reaction in anhydrous solvent, add ethylene oxide to the reaction solution at 0°C, acidify with dilute sulfuric acid to pH< 1. After layering, add an appropriate amount of antioxidant, p...
Embodiment 2
[0041] Example 2. A kind of synthetic method of 2-thienylethylamine, its step is as follows:
[0042] (1) Preparation of 2-bromothiophene: In the presence of an organic solvent, add a brominating agent dropwise to thiophene, control the reaction temperature at 10°C, keep the temperature for 6 hours after the dropwise addition, separate layers, remove the solvent, and then distill under reduced pressure Collect the 47°C / 1.73Kpa fraction to obtain the product; the organic solvent is dichloroethane or toluene; the brominating agent is N-succinimide, pyridine hydrobromide, bromine or hydrobromic acid Two kinds of; The molar ratio of substance is: thiophene: brominating agent=1: 3.0;
[0043] (2) Preparation of 2-thiophene ethanol: take 2-bromothiophene and magnesium chips to undergo Grignard reaction in anhydrous solvent, add ethylene oxide to the reaction solution at 20°C, acidify with dilute sulfuric acid to pH< 1. After layering, add an appropriate amount of antioxidant, remove...
Embodiment 3
[0045] Example 3. A kind of synthetic method of 2-thienylethylamine, its step is as follows:
[0046] (1) Preparation of 2-bromothiophene: in the presence of an organic solvent, add a brominating agent dropwise to thiophene, control the reaction temperature to 0°C, keep the reaction for 4 hours after the dropwise addition, separate layers, remove the solvent, and then distill under reduced pressure Collect 46 ℃ / 1.73Kpa fraction to obtain product; Described organic solvent is acetonitrile; Described brominating agent is the mixture of bromine and hydrobromic acid, and the molar ratio of the two is 1: 1; The molar ratio of substance is: Thiophene:brominating agent=1:2.5;
[0047] (2) Preparation of 2-thiophene ethanol: take 2-bromothiophene and magnesium chips to undergo Grignard reaction in anhydrous solvent, add ethylene oxide to the reaction solution at 9°C, acidify with dilute sulfuric acid to pH< 1. After layering, add an appropriate amount of antioxidant, remove the solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com