Symmetrical double-rhodamine B fluorescent probe for detecting mercury ion and preparation method of fluorescent probe
A fluorescent probe, mercury ion technology, used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve high selectivity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of rhodamine B acid chloride: Rhodamine B (0.49g) and phosphorus oxychloride (0.5ml) were refluxed in 30ml of dichloroethane for 16 hours, and the solvent was removed by rotary evaporation. The obtained acid chloride was used in the next reaction .
[0027] (2) Synthesis of rhodamine B-acyl-2-bromoethylamine: the obtained rhodamine B-acyl chloride (0.5 g) and 2-bromoethylamine (0.3 g) were dissolved in 30 mL of acetonitrile, and stirred overnight at room temperature for reaction. Solvent was removed using a rotary evaporator. The obtained crude product was purified by silica gel column chromatography, and the eluting solvent was n-hexane and ethyl acetate in a volume ratio of 1:1.
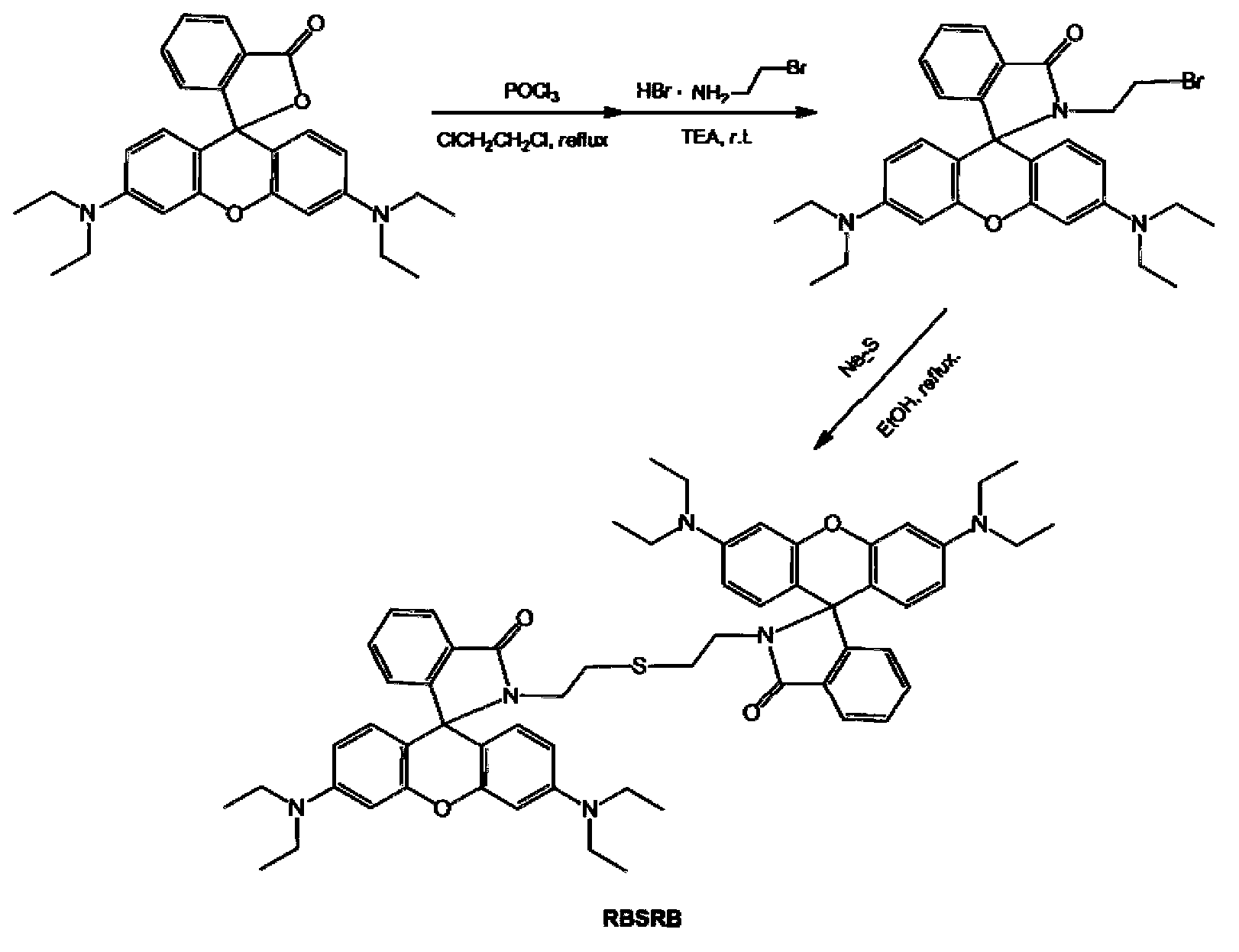
[0028] (3) Synthesis of the symmetrical bisrhodamine B fluorescent probe RBSRB: Reflux the obtained Rhodamine B-2-bromoethylamine (0.1g) and Na2S (0.5g) in 20ml ethanol for 6h, and remove the solvent with a rotary evaporator , purified by silica gel column chromatography, the...
Embodiment 2
[0032] The selectivity of the fluorescent probe RBSRB for the fluorescence detection of mercury ions:
[0033] Tris-HCl buffer solution with pH=7.4 was used to control the experimental conditions to meet the physiological environmental conditions.
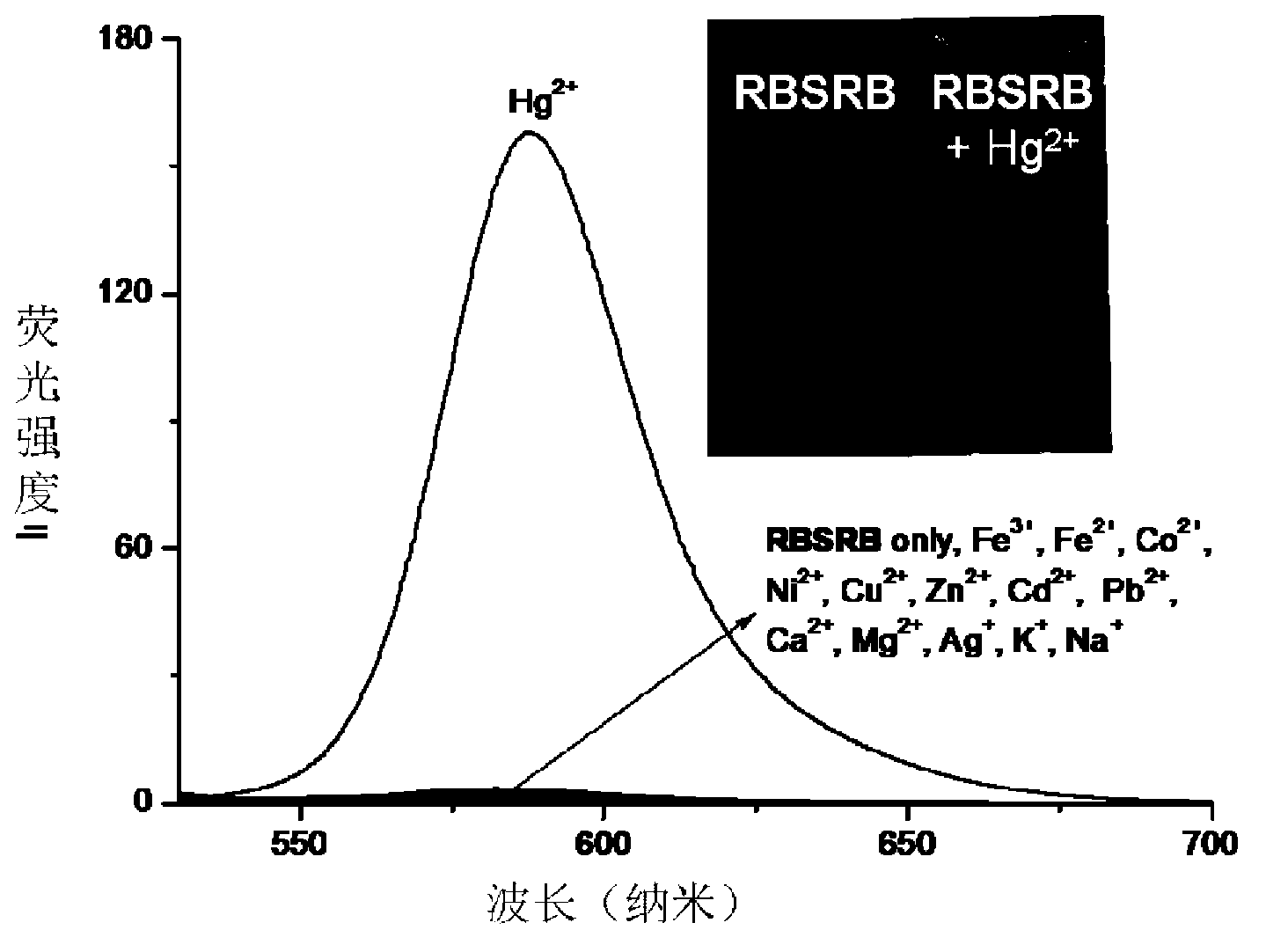
[0034] Add 0.1ml ethanol solution (1mM) of fluorescent probe RBSRB, 8.0ml Tris-HCl buffer solution, 0.3ml aqueous solution of metal ions to be tested to different 10ml colorimetric tubes (the concentration of mercury ions is 1mM, and the concentration of other metal ions Both are 10mM), and dilute to 10ml with Tris-HCl buffer solution. After constant volume, the concentration of fluorescent probe is 10 M, and the concentration of mercury ion is M, and the concentration of other metal ions was 300 M. Transfer 3ml of the working solution to a 1cm fluorescence cuvette to measure the fluorescence spectrum, the excitation wavelength is 510nm. In the blank experiment, no metal ions were added to the above solution. Selective detecti...
Embodiment 3
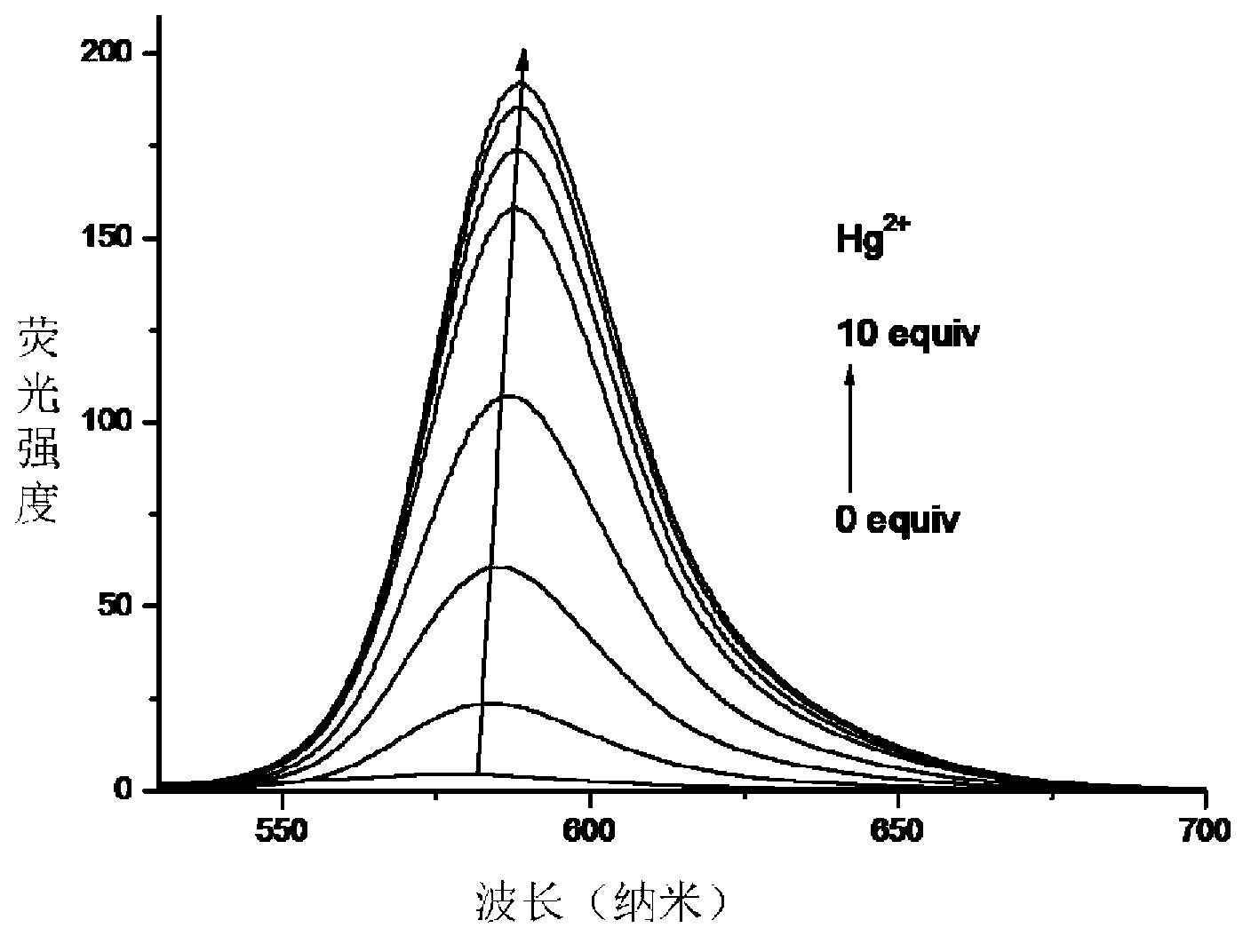
[0036] Quantitative fluorescence detection of mercury ions by fluorescent probe RBSRB:
[0037] Add 0.1ml ethanol solution (1mM) of fluorescent probe 1, 8.0ml Tris-HCl buffer solution, 0-1.0ml different volumes of 1mM mercury ion aqueous solution to different 10ml colorimetric tubes, and use Tris-HCl buffer solution to make up the volume to 10ml. After constant volume, the concentration of fluorescent probe is M, the mercury ion concentration is 0-100 M. Transfer 3ml of the working solution to a 1cm fluorescence cuvette to record the fluorescence spectrum and read and record the fluorescence intensity at 588nm. The fluorescence intensity and the corresponding mercury ion concentration data were input into the software Origin8 for fitting, and a linear working curve was obtained in the range of mercury ion concentration 0-30 M, and the linear regression constant was 0.9957, which indicated that the probe could quantitatively detect the concentration of mercury ions. The cha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com