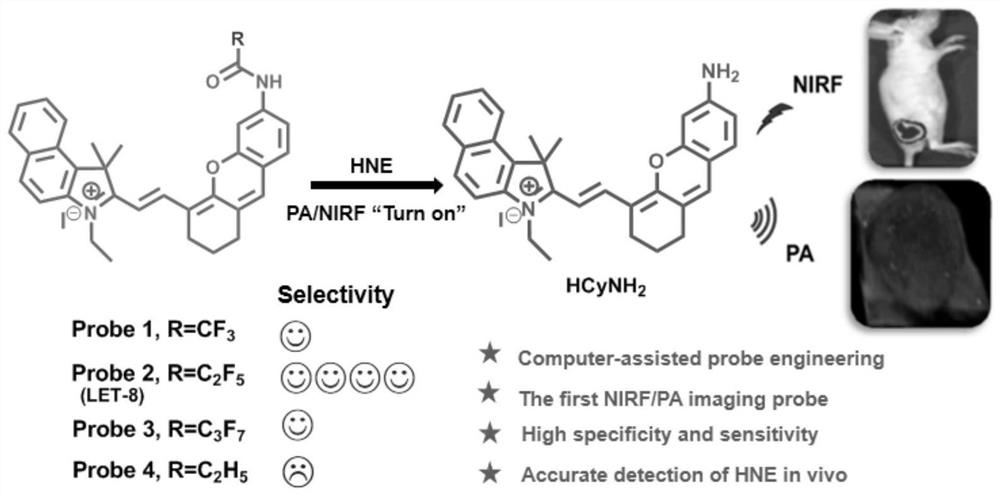
Molecular probe for dual-mode imaging detection of neutrophil elastase as well as preparation method and application of molecular probe
A technology of elastase and neutrophils, applied in the field of biomedical detection, can solve the problems of ELISA with many influencing factors, low efficiency, and high-throughput testing that cannot be performed by chromatography
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
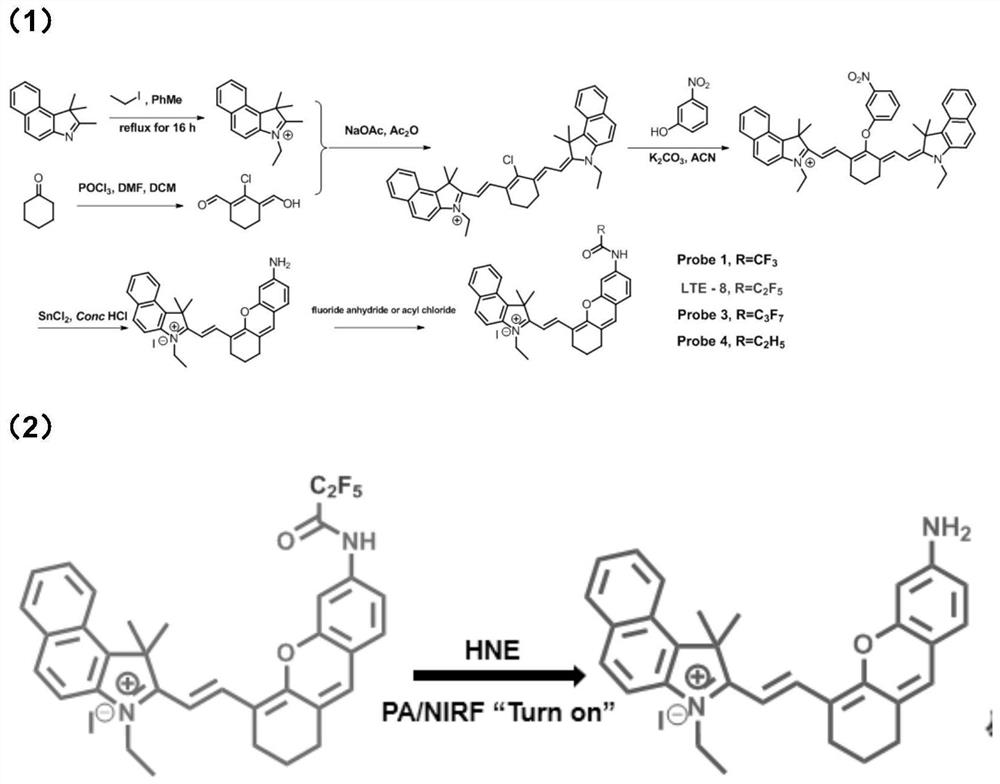
[0076] as attached figure 2 Shown in (1), first prepare Cy7-Cl according to the shown route. Then 350mg of 3-nitrophenol and 350mg of potassium carbonate were dissolved in 10mL of acetonitrile and reacted at room temperature for 30min. 740 mg of Cy7-Cl was dissolved in 5 mL of acetonitrile and the solution was injected into the above mixture through a syringe, and the reaction mixture was reacted at room temperature for 4 h. The solvent was removed by rotary evaporation under reduced pressure, and the precipitate was dissolved in dichloromethane, washed with water three times, and dried over anhydrous sodium sulfate. The solvent was removed by rotary evaporation under reduced pressure to obtain the intermediate product Cy7-NO 2 .
[0077] The above intermediate product Cy7-NO 2 Dissolve in 30 mL of methanol, and add 3.8 g of stannous chloride dissolved in 4 mL of concentrated hydrochloric acid to the above solution. The reaction solution was heated to 70°C and stirred ov...
Embodiment 2
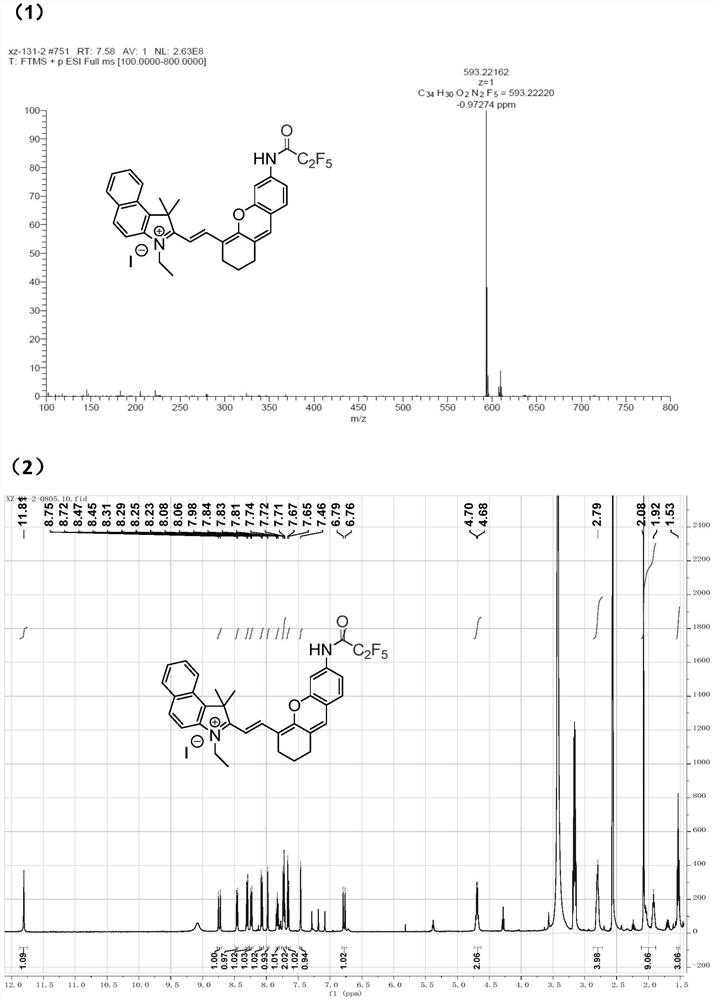
[0084] as attached image 3 As shown in (1), LET-8 was dissolved in methanol, and the HR-MS mass spectrum was obtained, and its mass-to-charge ratio was measured to be 593.22.
[0085] as attached image 3 As shown in (2), 5 mg of LET-8 was dissolved in 500 μL of deuterated methanol to obtain a proton nuclear magnetic resonance spectrum.
[0086] as attached image 3 As shown in (3), Probe-1 was dissolved in methanol to obtain an HR-MS mass spectrum, and its mass-to-charge ratio was measured to be 543.22.
[0087] as attached image 3 As shown in (4), 5 mg of Probe-1 was dissolved in 500 μL of deuterated methanol, and the H NMR spectrum was obtained.
[0088] as attached image 3 As shown in (5), Probe-3 was dissolved in methanol, and the HR-MS mass spectrum was obtained, and its mass-to-charge ratio was measured to be 643.21.
[0089] as attached image 3 As shown in (6), 5 mg of Probe-3 was dissolved in 500 μL of deuterated methanol, and the H NMR spectrum was obtaine...
Embodiment 3
[0093] LET-8 was dispersed in a mixed solvent (PBS / DMSO=95 / 5, v / v), and prepared into 5uM and 10μM LET-8 solutions for fluorescence detection and photoacoustic detection, respectively.
[0094] as attached Figure 4 As shown in (1), two groups of LET-8 solutions were taken, the first group was not treated, the second group was incubated with 0.6 μg / mL HNE at 37°C for 60 min, and the color changes of the solutions before and after the reaction were photographed and recorded.
[0095] as attached Figure 4 As shown in (2), take 10 μM LET-8 solution, add 0-0.6 μg / mL HNE, and record the changes before and after the UV-Vis spectrum.
[0096] as attached Figure 4 In (3), take 5 μM LET-8 solution, add 0-0.6 μg / mL HNE, and record the changes of its fluorescence emission spectrum before and after.
[0097] as attached Figure 4 In (4), 10 μM LET-8 solution was taken, and 0-0.6 μg / mL HNE was added to record the change of its photoacoustic spectrum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com