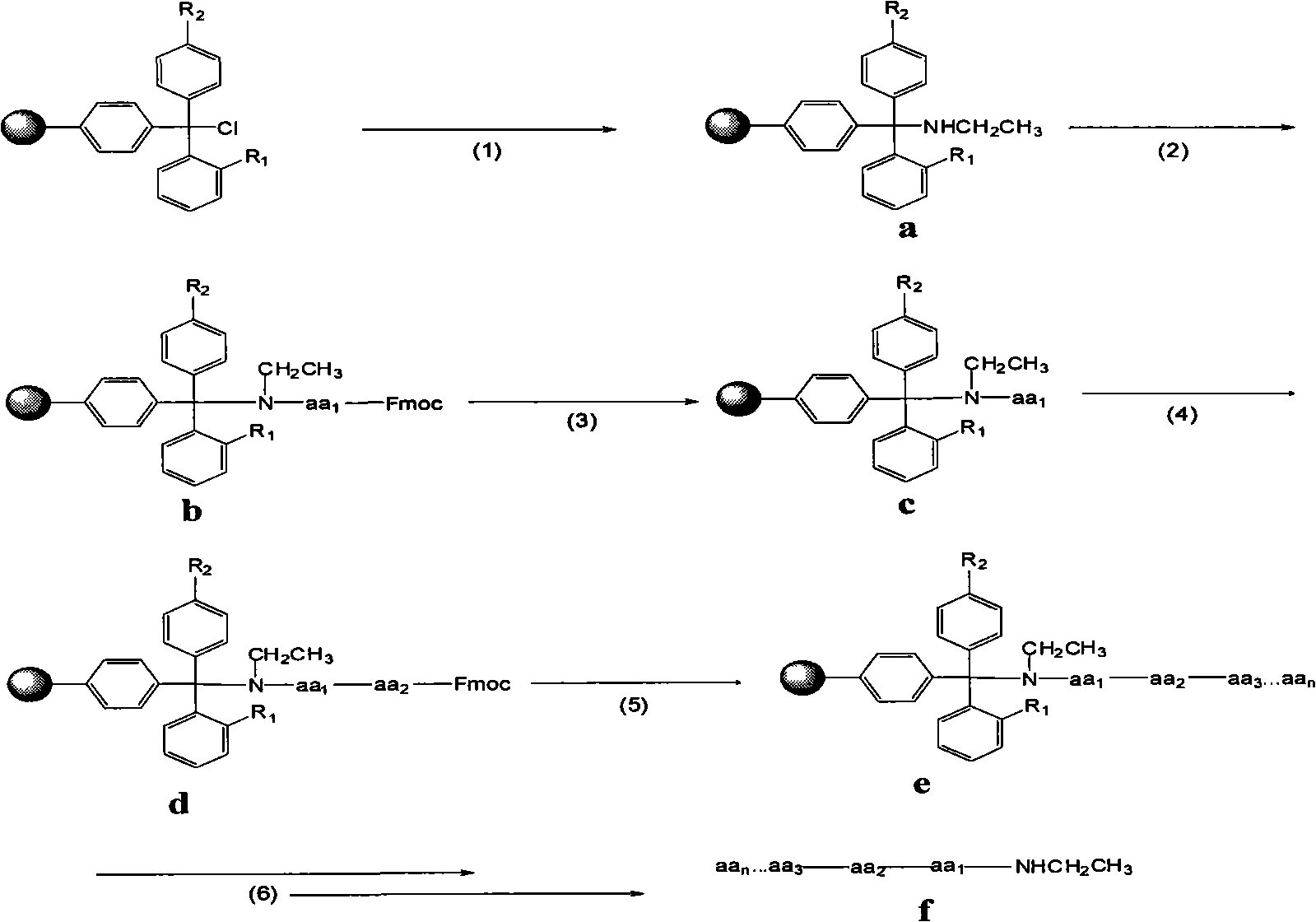
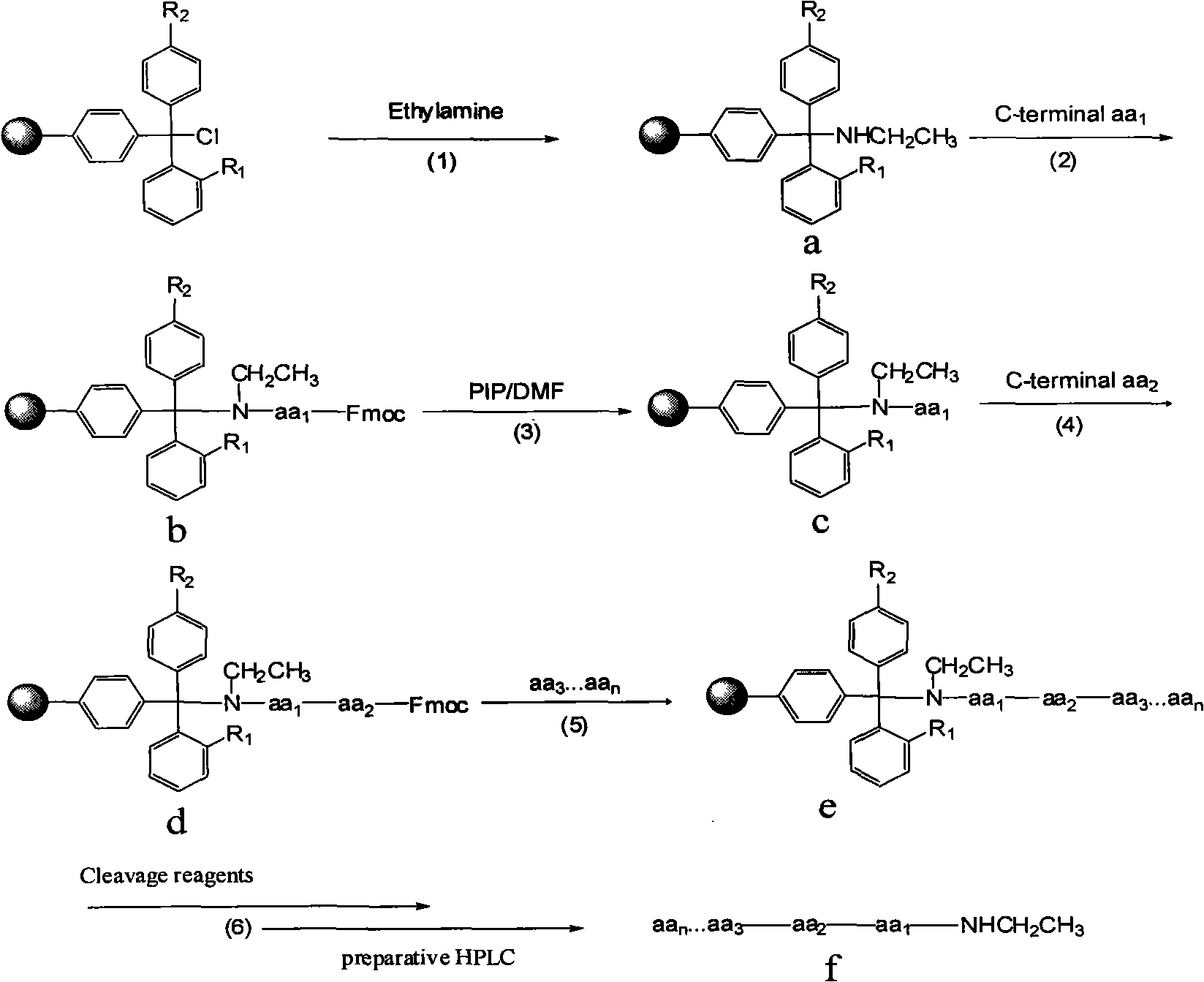
Preparation of C-terminal ethylamine polypeptides and derivates thereof
A derivative, the technology of terminal ethylamine, applied in the field of polypeptide chemistry, can solve the problems of cumbersome process, difficult to scale up, pollution costs and other problems, and achieve the effect of simple process, reduced preparation cost and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Preparation of leuprolide (pGlu-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-Pro-NHEt) (1) Preparation of ethylamine resin: weighing 2-chlorotrityl Put 1 g of chlorine resin (1 mmol / g) in a glass sand core reactor, add 15 mL of DCM, and let stand to swell the resin for 0.5 h. Introduce 0.326mL ethylamine gas. React at 10°C for 1h. After the reaction, filter. Then 15 mL of DMF was added to the resin to wash the resin and filtered. Then add 15mL THF to wash the resin, and filter.
[0023] (2) Connect the C-terminal amino acid Pro
[0024] Weigh 2 mmol each of Fmoc-Pro-OH, HBTU and HOBt, and add 10 mL of DMF to dissolve. Then the mixed solution was added to the resin in (1), and 4 mmol of diisopropylethylamine (DIEA) was added at the same time, and reacted at 20° C. for 1 h. After the reaction, filter. Then 15 mL of DMF was added to the resin to wash the resin and filtered. Add 15 mL of NMP to wash the resin and filter.
[0025] (3) Remove the Pro amino protecting ...
Embodiment 2
[0033]Example 2: Preparation of histrelin (Pyr-His-Trp-Ser-Tyr-D-His(Bzl)-Leu-Arg-Pro-NHEt)
[0034] (1) Preparation of ethylamine resin: Weigh 2 g of 4-methyl-2-chlorotrityl chloride resin (1 mmol / g), place it in a glass sand core reactor, add 20 mL of DMF, and let stand to swell the resin for 1 h . Introduce 0.653mL ethylamine gas. Reaction at 30°C for 2h. After the reaction, filter. 20 mL of DCM was then added to the resin to wash the resin and filtered. Another 20 mL of DCM was added to wash the resin and filtered.
[0035] (2) Connect the C-terminal amino acid Pro
[0036] Weigh Fmoc-Pro-OH, HATU and HOAt each 8mmol, 10mmol, 10mmol, add 20mL DMF to dissolve. Then the mixed solution was added to the resin in (1), and 16 mmol DIEA was added at the same time, and reacted at 40° C. for 5 h. After the reaction, filter. 20 mL of NMP was then added to the resin to wash the resin and filtered. Add 20 mL of NMP to wash the resin, and filter.
[0037] (3) Remove the Pro a...
Embodiment 3
[0045] Example 3: Preparation of Alarelin (pGlu-His-Trp-Ser-Tyr-D-Ala-Leu-Arg-Pro-NHEt)
[0046] (1) Preparation of ethylamine resin: Weigh 3 grams of 2-chlorotrityl chloride resin (1 mmol / g), place it in a glass sand core reactor, add THF 20 mL, and let stand to swell the resin for 2 hours. Introduce 1.306mL ethylamine gas. Reaction at 50°C for 3h. After the reaction, filter. 20 mL of DCM was then added to the resin to wash the resin and filtered. Another 20 mL of DCM was added to wash the resin and filtered.
[0047] (2) Connect the C-terminal amino acid Pro
[0048] Weigh 15mmol, 22.5mmol and 22.5mmol of Fmoc-Pro-OH, PyBOP and HOBt respectively, and add 25mL NMP to dissolve. Then the mixture was added to the resin in (1), and 30 mmol DIEA was added at the same time, and reacted at 60° C. for 10 h. After the reaction, filter. 20 mL of NMP was then added to the resin to wash the resin and filtered. Add 20 mL of DMF to wash the resin, and filter.
[0049] (3) Remove t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com