Tjn molecular kit for diagnosing virulent strains of helicobacter pylori
A Helicobacter pylori and kit technology, applied in the field of molecular diagnostic kits used to detect virulent strains of Helicobacter pylori, can solve problems such as difficult to use, and achieve high reliability, consistency and probability of definite results high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: The kit is applied to gastric biopsy samples
[0092] This is not a limiting example of the technology, but only a specific application of the kit developed.
[0093] sample:
[0094] Fifty-six biopsies were analyzed from the gastric antrum and gastric body of 26 patients who had seen a doctor for gastric and duodenal problems. Samples of H. pylori strains ATCC 43504, ATCC 25695 and 96.978 were used as positive controls and samples of human genomic DNA were used as negative controls.
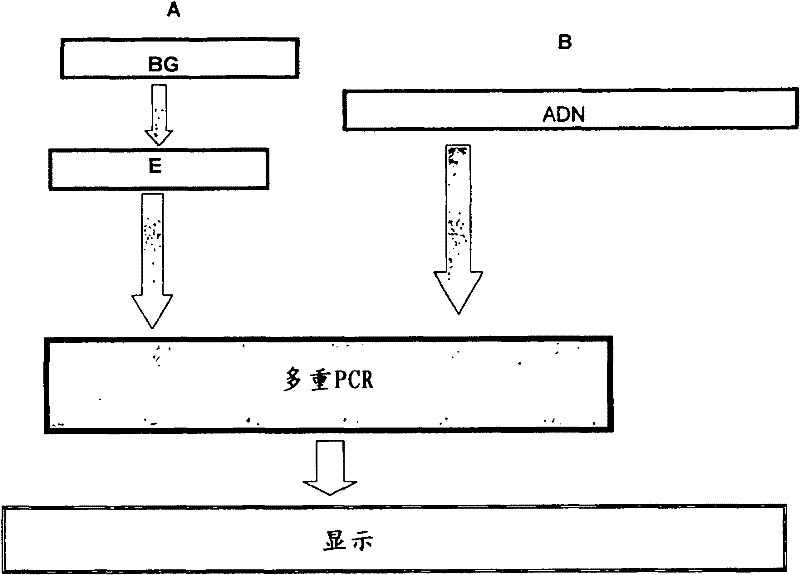
[0095] 1- First DNA extraction phase and secondary phases:
[0096] DNA was extracted using the reactions described below (available in the proposed kit): lysis buffer (TrisHCl 10 mM, EDTA 1 mM, SDS 10%); proteinase K (20 mg / mL); CTAB / NaCl solution; chloroform:iso Pentanol (24:1); Benzene:chloroform:isoamyl alcohol=25:24:1; ethanol and TE buffer. Fifty biopsies analyzed were included in the assay; other samples were used as controls and DNA was extracted from pure isolates ...
Embodiment 2
[0102] Embodiment 2: the comparison of the present invention and commercial kit
[0103] Our technology was compared with an alternative commercial kit MPCR H. pylori kit Cat N°. MP-700081. The latter showed significant differences in terms of results, as described below:
[0104] 1) The latter does not contain the reactions or steps required to extract DNA from gastric biopsy samples and / or H. pylori cultures.
[0105] 2) The latter does not provide the reactants Taq polymerase and dNTP, which means that kit users must prepare these substances in advance. Furthermore, it includes reactants in the form of sterile liquids, which are prone to loss of activity during temperature changes (they must be stored at -20°C and shipped at -20°C). Our invention includes a choice of two formats: sterile lyophilized beads (completely stable for storage and transport at ambient temperature) or sterile liquid.
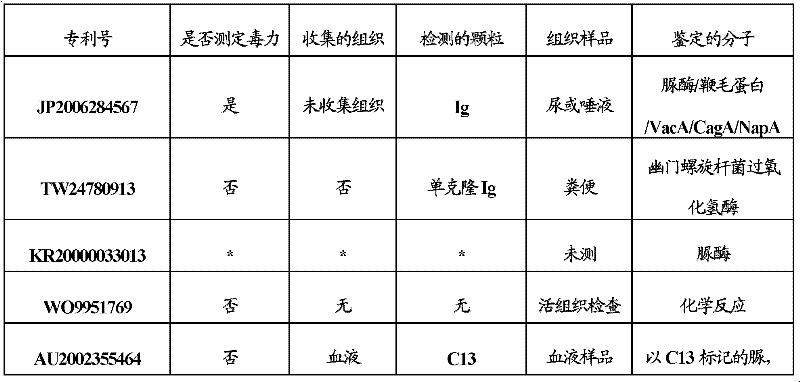
[0106] 3) MPCR Helicobacter pylori kit Cat N°.MP-700081 detects gene ureA, acc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com