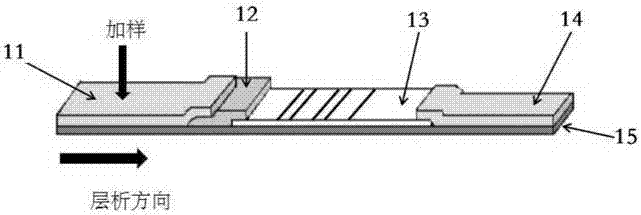
Test strip and kit for helicobacter pylori colloidal gold typing detection
A technology for detecting Helicobacter pylori and test paper, which is applied in the field of medical biology, can solve the problems of low sensitivity, long time consumption, radioactivity in the human body, etc., and achieves the effect of simple operation method and guaranteed reliability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Core antigen expression of Helicobacter pylori cytotoxicity (CagA), vacuolar toxin (VacA), urease subunit A protein and urease subunit B protein
[0036]The four gene sequences of Helicobacter pylori cytotoxicity (CagA), vacuole toxicity (VacA), urease subunit A protein and urease subunit B protein are the sequences of Helicobacter pylori strain 26695 collected by the applicant according to GenBank, after Preferably, the found core antigen sequence is artificially synthesized by Jinkai Rui Company. Wherein, the gene sequence of HP cytotoxicity (CagA) is Seq ID No.1, and the gene sequence of HP vacuolar toxin (VacA) is Seq ID No.2, and the gene sequence of the HP urease subunit A protein antigen is Seq ID No.2. ID No.3, the gene sequence of HP urease subunit B is Seq ID No.4.
[0037] 1.1 Artificial synthesis of the above four gene sequences
[0038] And add restriction site fragments at both ends of the gene as needed
[0039] 1.2 Construction of expressio...
Embodiment 2
[0048] Embodiment 2 detects the preparation of chromatographic membrane
[0049] 2.1. Preparation of coating buffer
[0050] KC10.2g, Na 2 Helicobacter pylori O 4 12H 2 O 2.9g, KH 2 PO 4 0.2g, methanol 30ml, distilled deionized water to 1000ml, 0.22um membrane filtration
[0051] 2.2 Preparation of nitrocellulose chromatography membrane
[0052] T line (detection line):
[0053] Dilute CagA, VacA, UreA and UreB core antigen proteins with coating buffer to 50-100ng / ml, adjust the machine, draw four detection lines (T lines) from the end of the gold standard pad, respectively CagA, VacA, UreB and UreA.
[0054] C line (quality control line):
[0055] Dilute the rabbit anti-mouse IgG antibody to 50-100 ng / ml with coating buffer, adjust the machine, draw the C line, which is the quality control line, and the C line is close to the absorbent pad, about 3mm away from the absorbent pad. The distance between the two lines is 5-8mm, and the lines should be fine and uniform. ...
Embodiment 3
[0056] Example 3 Preparation of gold-labeled anti-human IgG Fc segment monoclonal antibody
[0057] 3.1. Preparation of required solutions
[0058] (1) prepare chloroauric acid solution
[0059] 10g chloroauric acid; double distilled deionized water to 1000ml. The prepared solution was stored at 4°C.
[0060] Formulated 1% Trisodium Citrate
[0061] Dissolve trisodium citrate in double-distilled deionized water, filter through a 0.22um membrane, and prepare and use immediately.
[0062] Prepare 0.1Mol / L Potassium Carbonate
[0063] Prepare with double-distilled deionized water, 13.8g of potassium carbonate; dilute to 1000ml with double-distilled deionized water, filter with 0.22um membrane, and store at 4°C.
[0064] Formulated 2% PEG-20000
[0065] 20g PEG-20000 double-distilled deionized water to 1000ml, filter with 0.22um membrane, and store at 4°C.
[0066] Prepare labeling wash preservation solution
[0067] 2% bovine serum albumin (BSA), 0.05% sodium azide (NaN3)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com