Method for using lactoferrin for treatment or prevention of helicobacter pylori infection
A technology of Helicobacter pylori and lactoferrin, applied in peptide/protein components, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of long treatment course, inconvenient taking, lack of methods for Helicobacter pylori, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Embodiment 1 Helicobacter pylori cultivation and cryopreservation
[0153] Helicobacter pylori (Hp) culture: Weigh 3.9g of Columbia agar, dissolve in deionized water, dilute to 90ml, autoclave (121°C, 30min), add 10ml heat inactivation after cooling to 50-60°C Fetal bovine serum (56°C, 30min), Amphotericin B 200μl (5.0mg / ml), Vancomycin 100μl (10.0mg / ml), Polymyxin B 10μl (3.8mg / ml), TMP (5.0mg / ml ) 100 μl, mixed evenly and poured into a sterile culture dish to make a solid culture plate of Hp. The frozen standard HpSS1 strain (ATCC43504) was thawed on ice, inoculated on the Hp solid culture plate, and the culture dish was placed in an anaerobic culture box, and the microaerophilic (5% O 2 , 10% CO 2 and 85%N 2 ), 90% humidity, and cultured at 37° C. for 3 to 5 days. The well-growing Hp was transferred to a new medium for subculture, and cultured under the same conditions for 3 to 5 days.
[0154] Cryopreservation of Hp: The cryopreservation solution is 3.8% brain ...
Embodiment 2
[0155] The identification of embodiment 2 Helicobacter pylori
[0156] Identification of Hp: identification by two methods
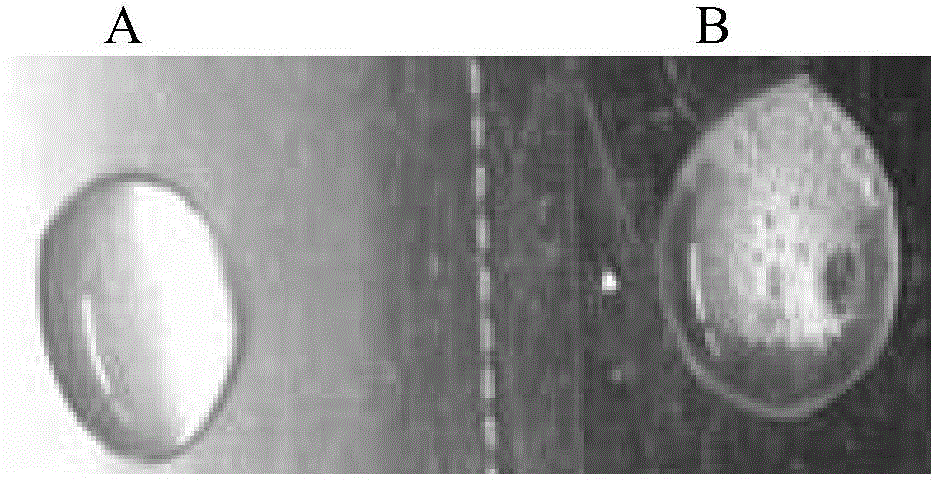
[0157] (1) Catalase experiment: Hp is a bacterium with catalase, which can catalyze hydrogen peroxide into water and oxygen, and bubbles appear. Catalyst test shows, the bacterium that is cultivated can make 3% hydrogen peroxide solution produce a large amount of bubbles (specifically as figure 1 shown).
[0158] (2) Rapid urease test:
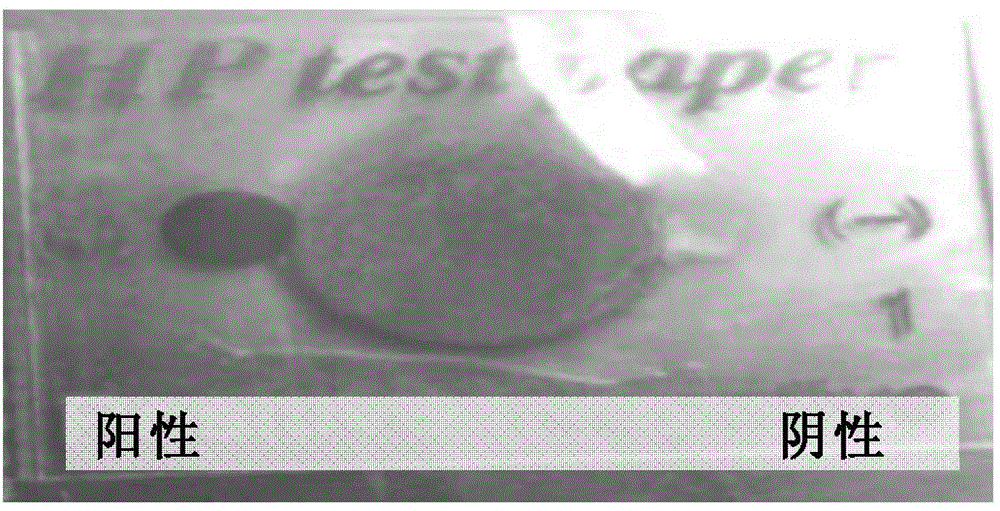
[0159] Helicobacter pylori can produce urease and divide urea to form ammonia gas, making the medium alkaline. Pick the culture of bacteria to be tested for 18 to 24 hours and inoculate it into gastric Helicobacter pylori (HP) test paper (Guangzhou Beisiqi Diagnostic Reagent Co., Ltd.) and continue to culture for 2 to 4 hours. The result is urease positive (specifically as figure 2 shown).
Embodiment 3
[0160] Embodiment 3. Plate culture method detects the effect of rhLF inhibiting Hp bacteria
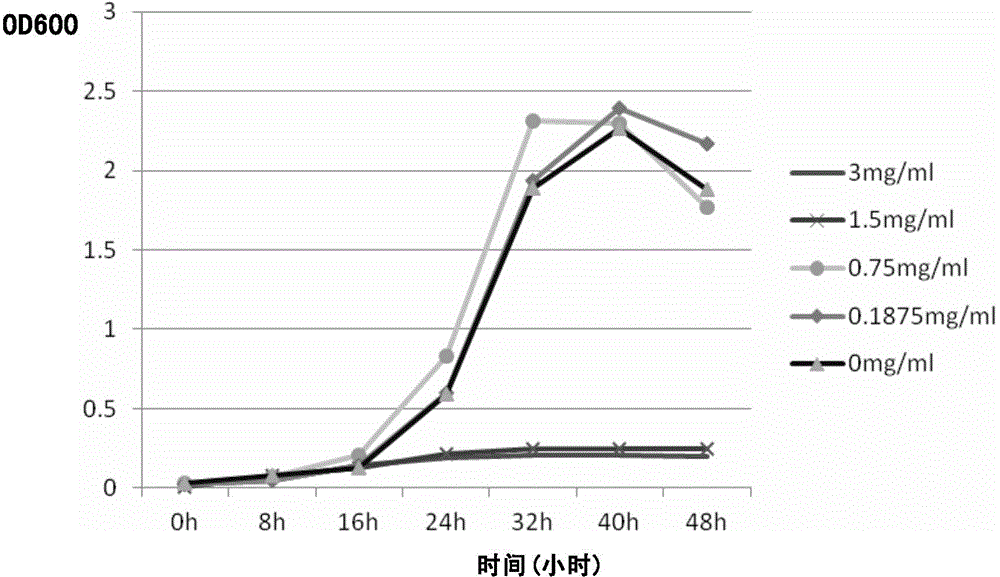
[0161]Take 6 sterile test tubes, add 6ml of 0.22μm filter-sterilized rhLF (15mg / ml) to the first tube, add 3ml of sterile water to the remaining tubes, and make 5 dilutions with the 2-fold ratio dilution method: 15, 7.5, 3.75, 1.875, 0.9375 mg / ml. The last tube without drug was used as negative control.
[0162] Take 6 sterile Erlenmeyer flasks, add 24.5ml / bottle of sterile Columbia culture, add 2.5ml / bottle of heat-inactivated fetal bovine serum, 3ml / bottle of rhLF and negative For the control, mix well and pour into a sterile Petri dish. In each group of plates, the concentration of rhLF was as follows: 1.5, 0.75, 0.375, 0.1875, 0.09375, 0 mg / ml. Plate No. 7 is a positive control with no bacteria and drugs.
[0163] Detect the OD600 value of Hp bacteria by spectrophotometer method, adjust the bacterial concentration to 1×108cfu / ml, take 100 μl of Hp bacteria suspension on the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com