Lithium-enriched anti-perovskite sulfides, solid electrolyte material containing lithium-enriched anti-perovskite sulfides and application of solid electrolyte material
A solid electrolyte and anti-perovskite technology, applied in electrolytes, circuits, electrical components, etc., can solve the problems of narrow electrochemical window, high environmental requirements, poor mechanical strength, etc., and achieve high charge and discharge rate and carrier concentration The effect of high, high total conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
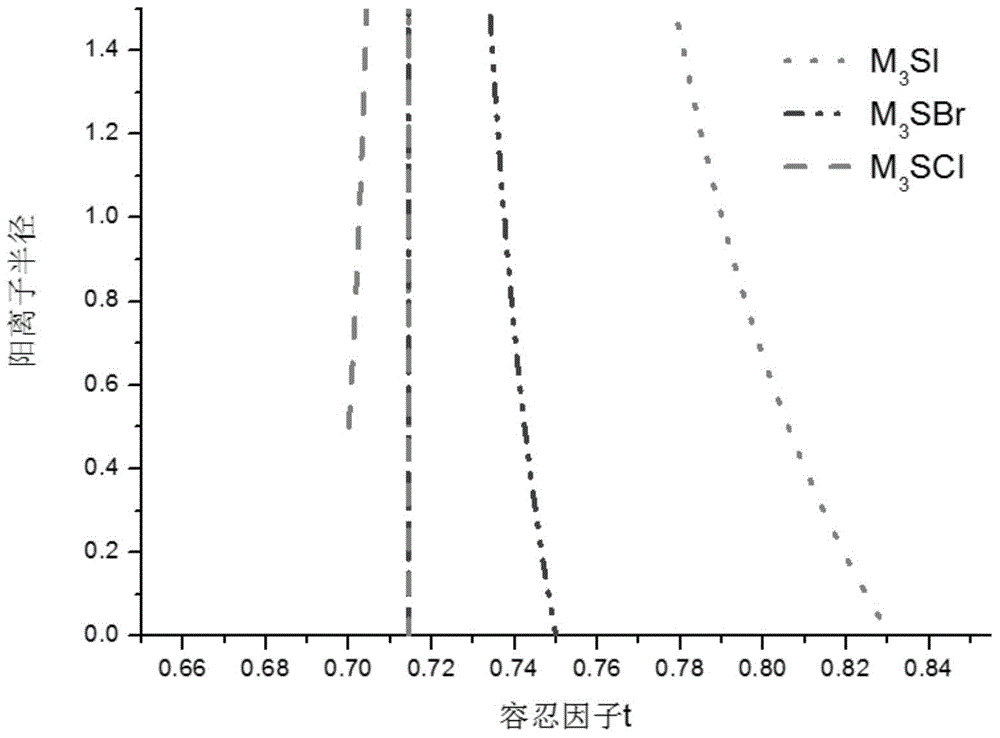
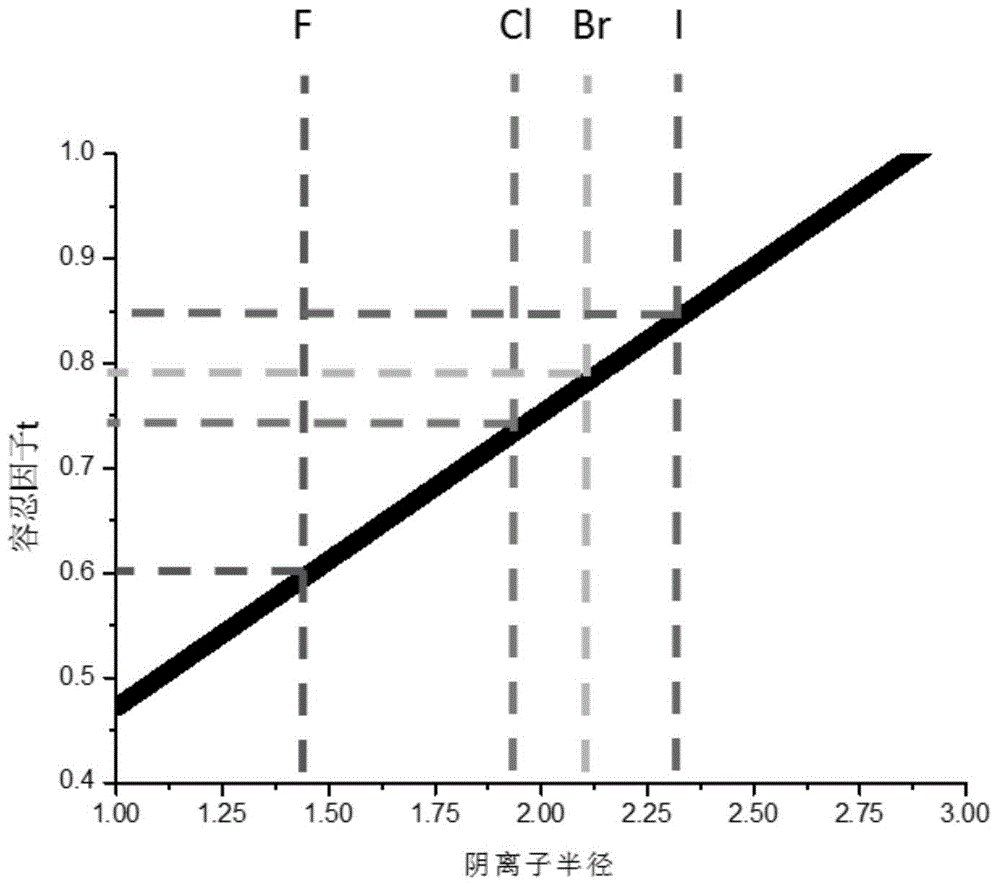
[0094] According to different preparation methods, the composite electrolyte material system provided by the present invention can be in a crystalline state, an amorphous state or a composite crystalline state. Whether the electrolyte material provided by the present invention is a pure phase, a single phase or a composite material, all of the following conditions can be met simultaneously, including: high ionic conductivity, negligible electronic conductance, wide electrochemical window, and electrode material Chemical matching (including no reaction with the electrode, and lower impedance at the interface with the electrode material), mutual matching with the stress and strain of the electrode material during the process of deintercalating lithium and thermal expansion, low raw material costs and little environmental pollution, and manufacturing Low cost and easy industrial production.
[0095] In addition to containing lithium-rich antiperovskite sulfide, the composite elec...
Embodiment 1
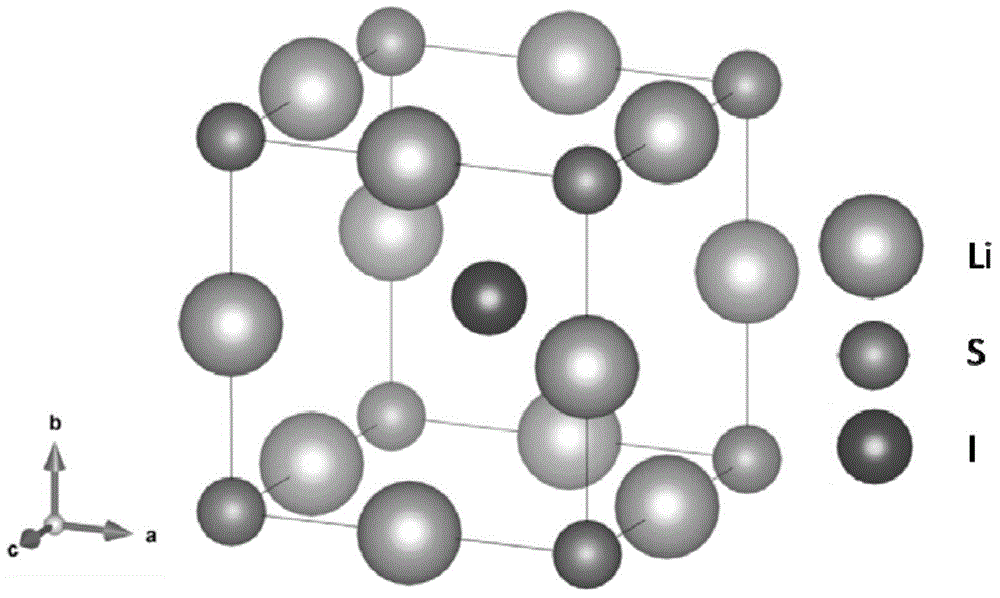
[0240] Embodiment 1[crystalline state: Li 2 S / LiBr=1 / 1]
[0241] Weigh 0.4595 g (0.01 mol) of lithium sulfide and 0.8685 g (0.01 mol) of lithium bromide and mix them uniformly in an argon atmosphere glove box. The mixed powder was transferred into a sealed ball mill jar in the glove box, and after it was completely sealed, it was removed from the glove box. The rotation of the planetary ball mill was low-speed rotation (100 rpm), and the mechanical grinding was performed for 20 minutes to fully mix the lithium sulfide and lithium iodide. Move the homogeneously mixed sample into a glove box, press it into a cylinder with a diameter of 5 mm and a thickness of 1 mm, and seal it with a gold capsule 1 cm long and 5 mm in diameter. Put the sealed capsule into the six-sided top press to synthesize the sample, the pressure is 5.0Gpa, the temperature is 300°C, and the heat preservation and pressure holding time is 30 minutes. After stopping the heating, it was cooled to room tempera...
Embodiment 2
[0242] Embodiment 2[amorphous form: Li 2 S / LiBr=1 / 1]
[0243] Weigh lithium sulfide 0.4595g (0.01mol) and lithium bromide 0.8685g (0.01mol), dissolve in denatured absolute ethanol solution (90% ethanol, 5% methanol, 5% isopropanol) in an argon atmosphere glove box, seal After that, stir at room temperature for 12 hours and mix well. The solution was transferred to a vacuum oven for drying. The obtained powder was evaluated by X-ray measurement, and vitrification (sulfide glass) was confirmed as a result. The conductivity is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com