Membrane-electrode assembly, electrolytic cell employing the same, electrolytic-water sprayer, and method of sterilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
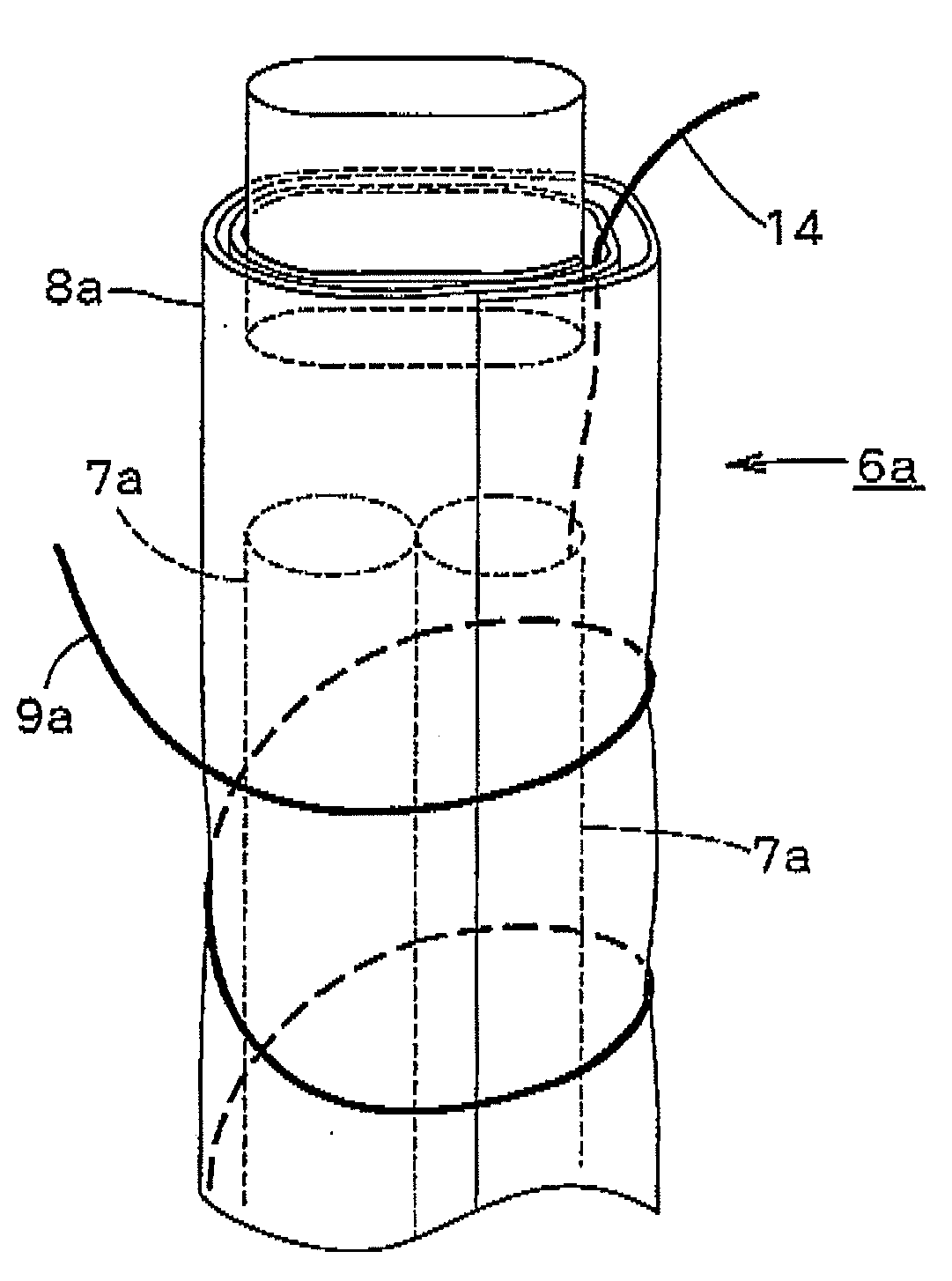
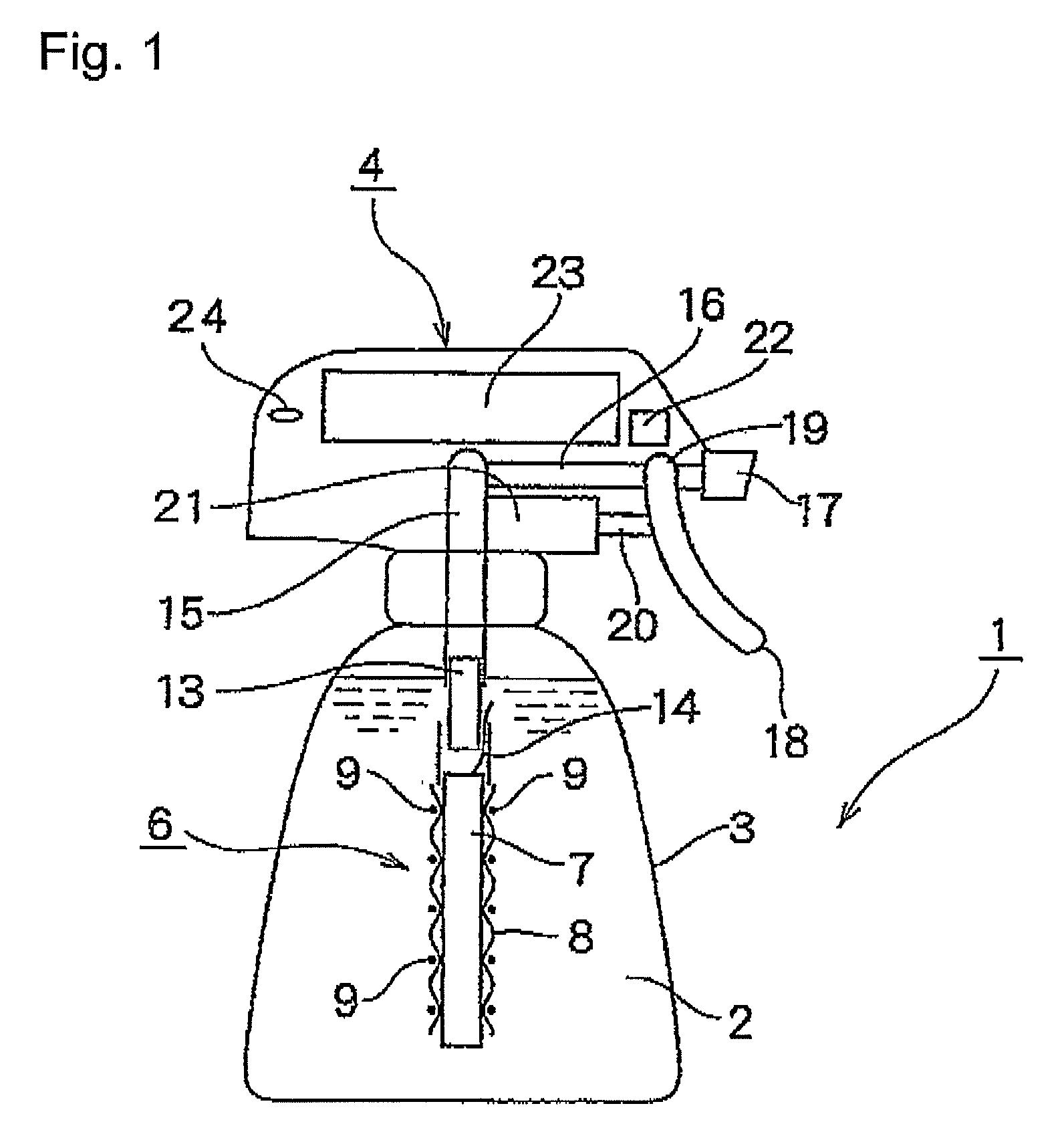
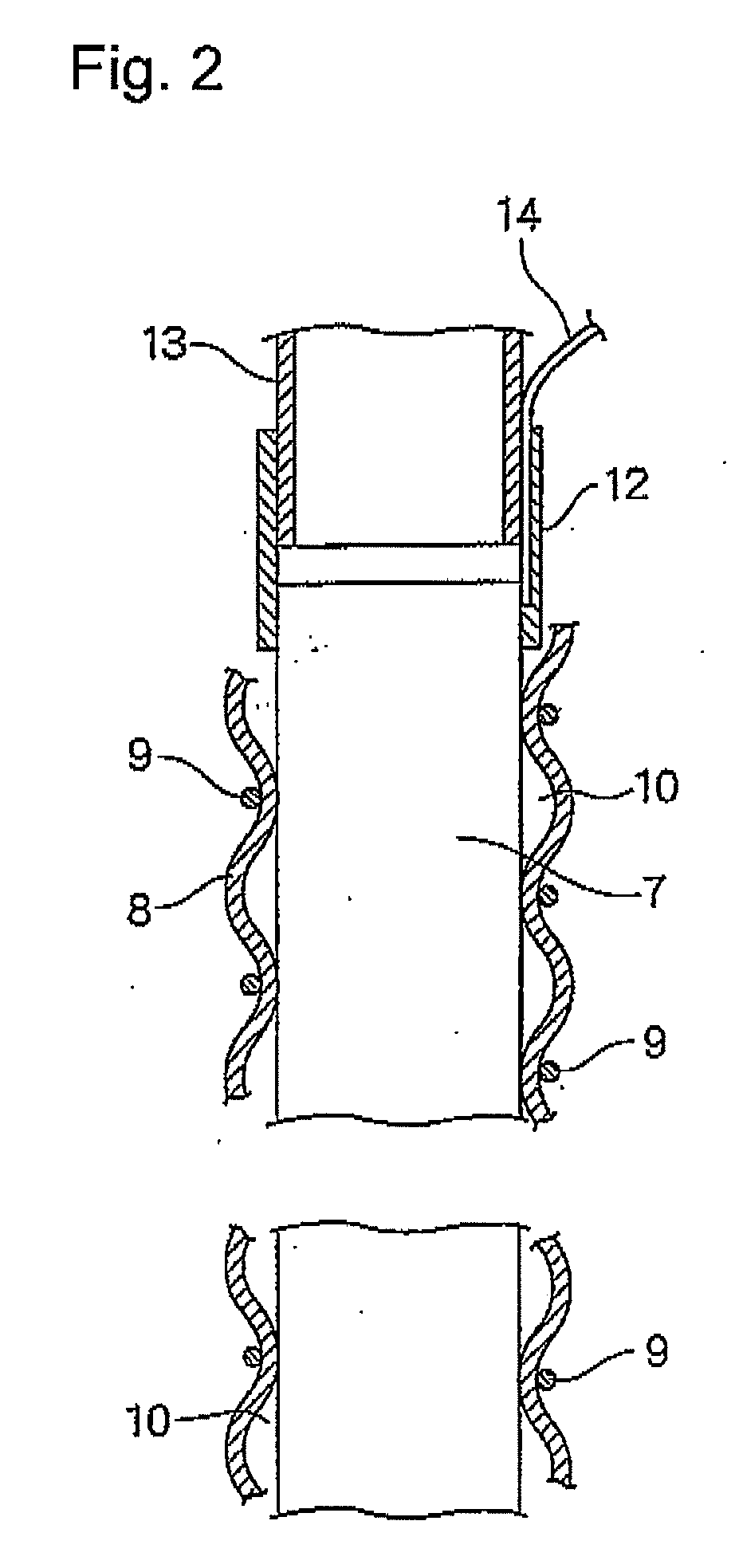
[0198]A rod made of niobium (diameter, 2 mm) on which a conductive-diamond catalyst (dopant boron concentration, 2,500 ppm) had been deposited was placed as an anode in a tubular ion-exchange membrane (Nafion 350, manufactured by DuPont; thickness, 0.35 mm; diameter, 3 mm). A commercial platinum wire (diameter, 0.4 mm) was spirally wound as a cathode on the diaphragm to obtain an anode-membrane-cathode assembly. The winding pitch was 4 mm. Tubes (diameter, 4 mm) were bonded to upper and lower parts of the assembly, and feeder wires from a DC power source were connected to the respective electrodes to obtain an electrolytic cell. Pure water was passed upward through the anode chamber at a rate of 40 cc / min. Currents of 0.5 A and 1 A were separately caused to flow. As a result, the cell voltages were 13 V and 19 V, respectively, the ozonized water concentrations were 8 ppm and 21 ppm, respectively, and the ozone generation efficiencies were 13% and 12%, respectively, in these operatio...
example 2
[0202]Those parts of the membrane in the assembly of Example 1 which constituted the two openings of the anode chamber were fixed to tubes having a diameter of 4 mm. The resultant member was disposed in a second tube having an inner diameter of 5 mm to form a cathode chamber between the second tube and the membrane. A feeder terminal for the wire cathode was connected to the cathode in the second tube. A 2 g / L aqueous solution of sodium chloride was fed to the anode chamber at a rate of 40 cc / min, and water was fed also to the cathode chamber in the same manner. A current of 1 A was caused to flow. As a result, alkaline water containing hydrogen and having a pH of 11 and acid water containing hypochlorite ions in an amount of 40 ppm could be simultaneously yielded.
example 3
[0203]The same test as in Example 2 was conducted, except that tap water was fed to the anode chamber and the cathode chamber. As a result, the ozonized water yielded at 0.5 A had a concentration of 4.5 ppm (current efficiency, 7.3%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com