Preparation method of injectable hydrogel precursor solution, injectable hydrogel and application of injectable hydrogel
A technology of hydrogel and precursor liquid, which is applied in the field of biomedicine, can solve the problem that the shape cannot meet people's needs, and achieve the effects of low drug delivery efficiency, good injectability, and high drug delivery efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation method of the injectable hydrogel precursor of the present embodiment comprises the following steps:
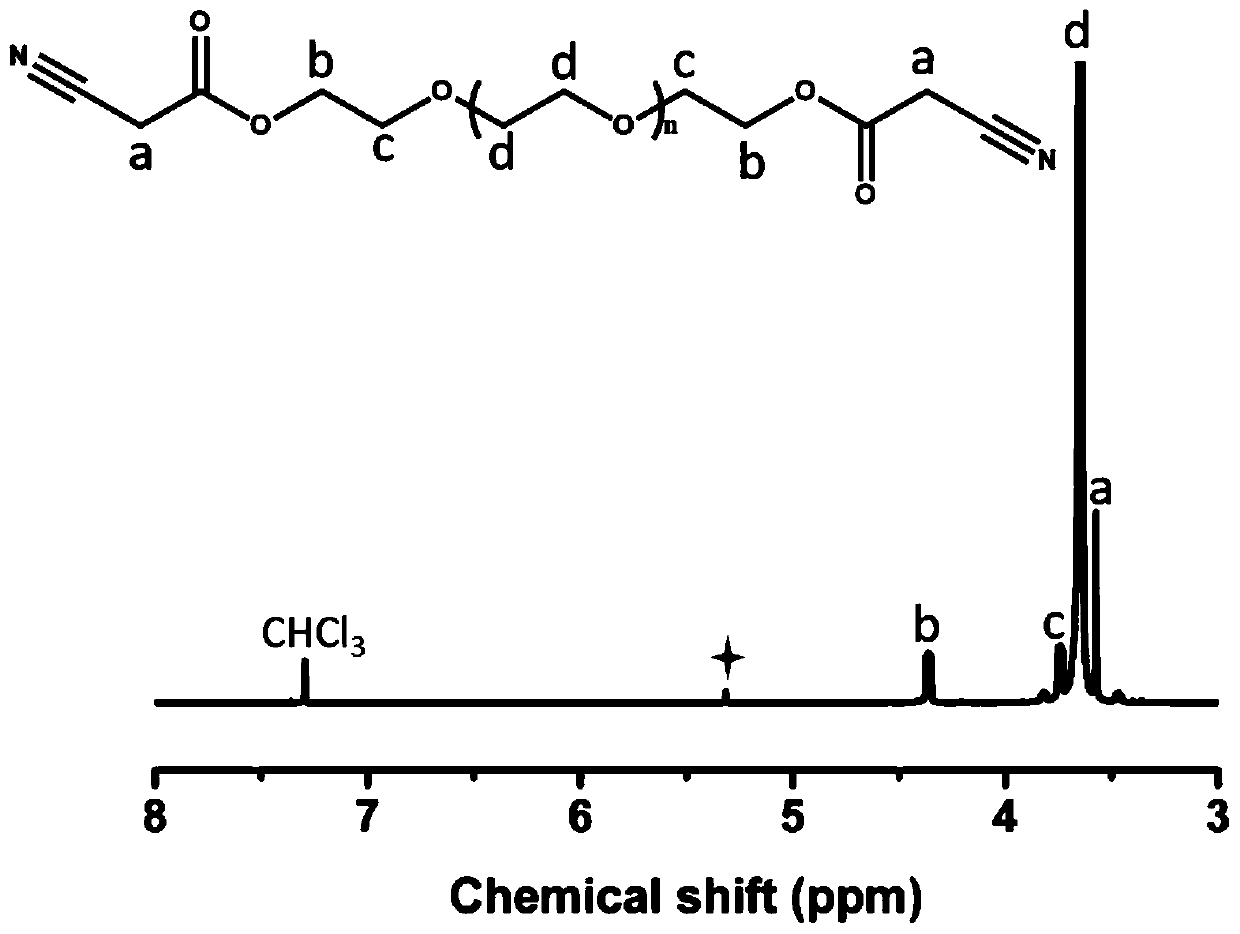
[0044] S1. Weigh 5g of PEG2000 and 1.08g of cyanoacetic acid, dissolve them in 80mL of benzene, then add 2-3 drops of concentrated sulfuric acid, and reflux at 100°C for 24 hours to obtain a light yellow liquid; After washing with sodium bicarbonate solution and drying with anhydrous magnesium sulfate, filter; the obtained filtrate was concentrated to obtain PEG2000 cyanoacetate with a yield of 80%. In this example, saturated sodium bicarbonate solution was used to wash with water three times to remove the reaction residue in the sample.
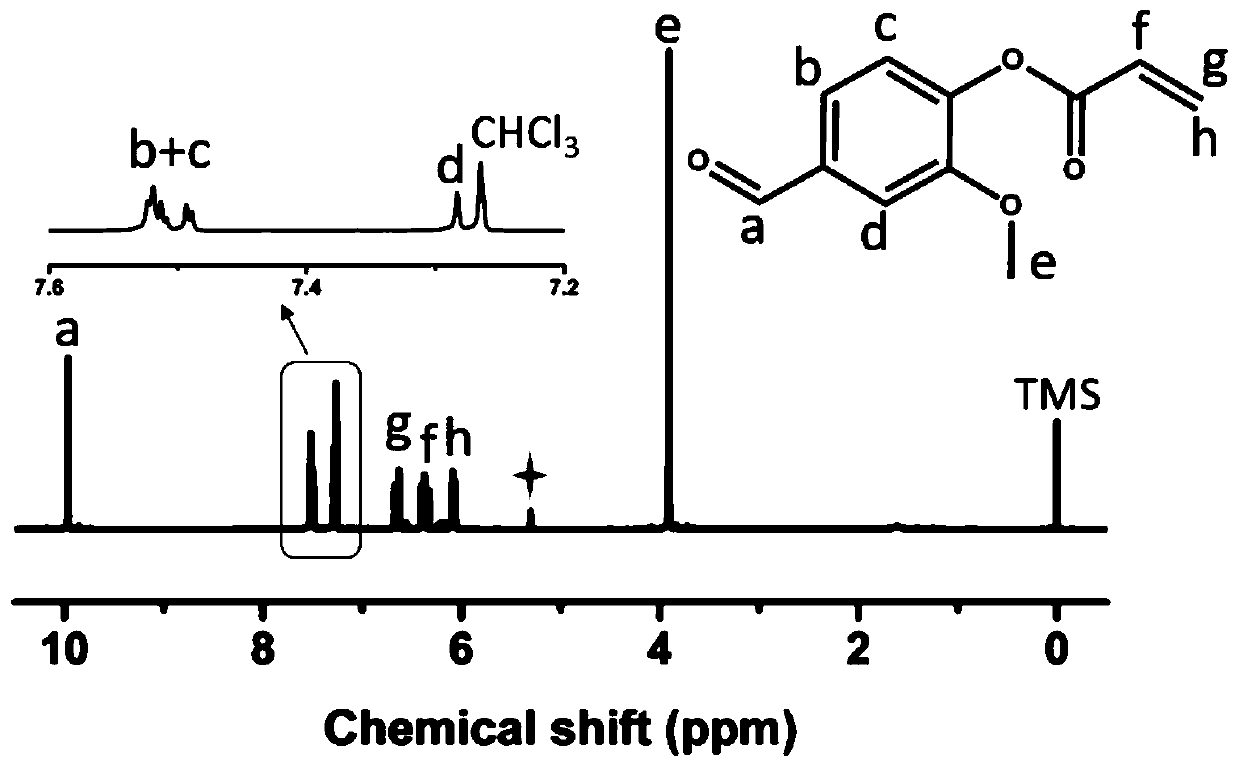
[0045] S2. Weigh 5g of vanillin in a flask, add 30mL of anhydrous dichloromethane, then add 4.94g of triethylamine, and stir the reaction in an ice bath; add 30mL of anhydrous dichloromethane into a constant pressure separatory funnel Methane, and then add 4.60g of acryloyl chloride, adjust the piston of the constant...
Embodiment 2
[0059] Same as Example 1, the difference is that the concentrations of the first solution and the second solution in step S4 are 20%. Dissolve PEG2000 cyanoacetate and water-soluble polyaldehyde respectively in PBS aqueous solution with pH=7.4 to obtain a first solution and a second solution with a concentration of 20%; quickly mix the first solution and the second solution evenly, and let stand , to produce a hydrogel.
Embodiment 3
[0061] Same as Example 1, the difference is that the concentrations of the first solution and the second solution in step S4 are 10%. Dissolve PEG2000 cyanoacetate and water-soluble polyaldehyde respectively in PBS aqueous solution with pH=7.4 to obtain a first solution and a second solution with a concentration of 10%; quickly mix the first solution and the second solution evenly, and let stand , to produce a hydrogel.
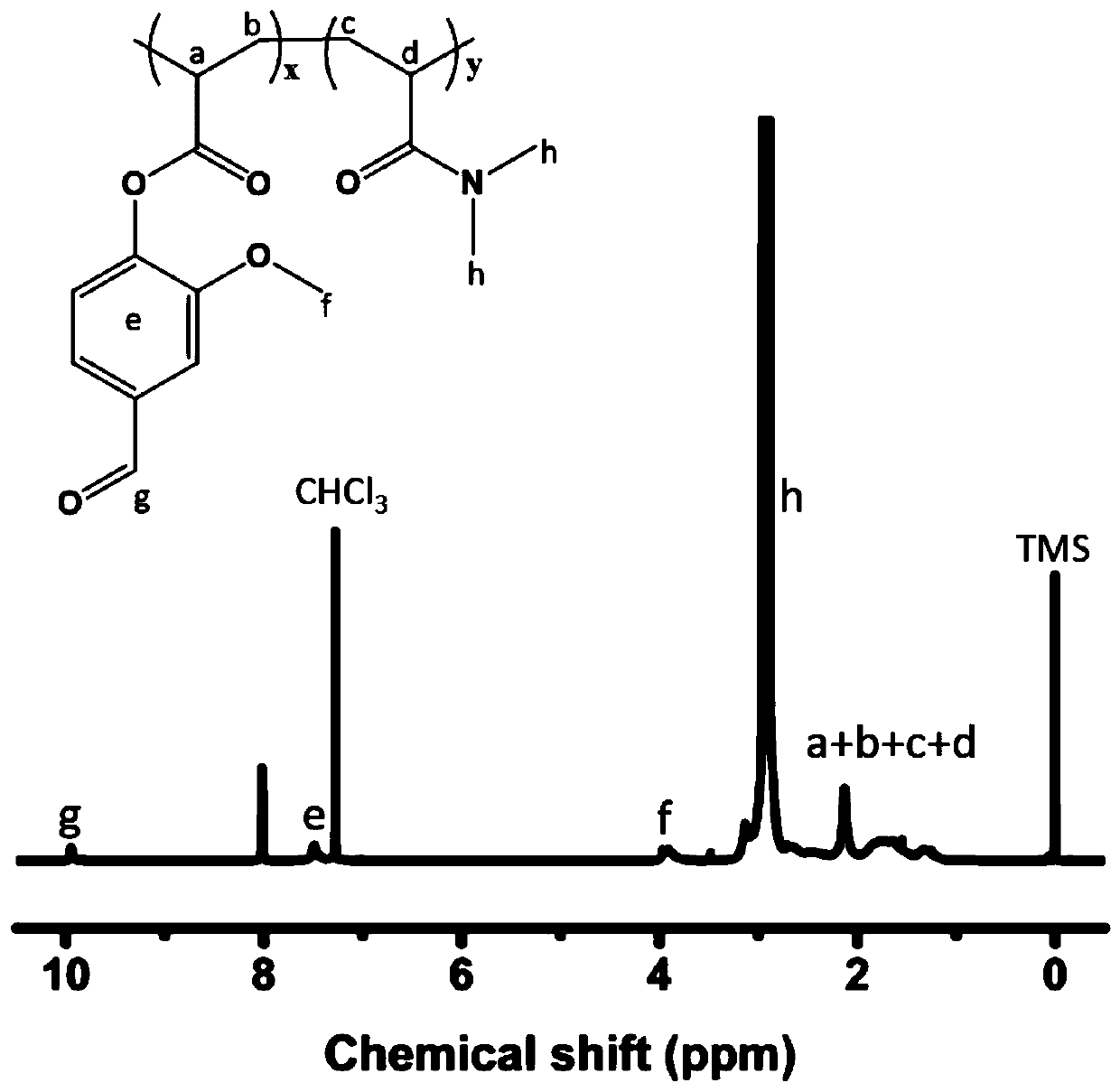
[0062] This application is based on the hydrogel formed by reversible dynamic covalent bonds. The active methylene group provided by PEG2000 cyanoacetate and the aldehyde group provided by water-soluble polyaldehyde are catalyzed by PBS and formed by Knoevenagel reaction. , which is also the first study to extend the Knoevenagel reaction to the preparation of hydrogel systems. And CN110522948A is a kind of hydrogel system based on hydrazide bond.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mechanical strength | aaaaa | aaaaa |
| Mechanical strength | aaaaa | aaaaa |
| Mechanical strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com