Oxynitride sealing glass and preparation method thereof
A technology for sealing glass and nitrogen oxides, which is applied in glass manufacturing equipment, glass molding, manufacturing tools, etc., can solve the problem of low nitrogen doping, and achieve the effects of stable preparation process, easy access to raw materials, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
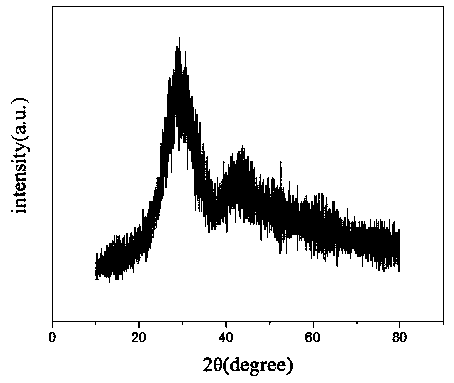
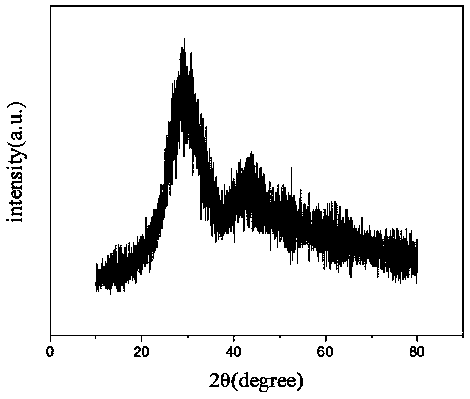
Image
Examples
Embodiment 1
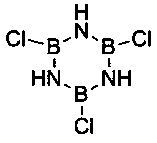
[0034] 1. Add 30 ml H to 2.13 g trichloroborazine 2 O, add 1 M / L NH dropwise 3 •H 2 0 to pH=8, stirred at 45 °C for 24 h to obtain solution A, and set aside;
[0035] 2. Take 4.92 g of ATSB, add 2.6 g of EAA, add IPA to make the overall weight 24 g, and stir for 1 hour to obtain solution B, set aside;
[0036] 3. Take 3.22 g of TMB, add IPA to the total mass of 24 g, stir for 1 h to obtain solution C, and set aside;
[0037] 4. Take TEOS 14.11 g, add H 2 O 2.82 ml, 1 M / mol HNO 3 2.83g, add IPA to make the overall mass 24g, magnetic stirrer for 1 h, obtain solution D, set aside;
[0038] 5. After mixing solutions B, C, and D and stirring them magnetically for 1 h, add 10.65 g of Sr(NO 3 ) 2 , after the complete dissolution, continue magnetic stirring for 30 min, add 11.88 g Ca(NO 3 ) 2 • 4H 2 O, after completely dissolving, stir for 30 min to obtain solution F;
[0039] 6. Add solution A dropwise to the obtained homogeneous solution F, and magnetically stir for 30 ...
Embodiment 2
[0045] 1. Add 50 ml H to 12.45 g trichloroborazine 2 O, add 1 M / L NH dropwise 3 •H 2 O to pH=8, stirred at 45 °C for 24 h, A obtained solution A, set aside;
[0046] 2. Take 4.92 g of ATSB, add 2.6 g of EAA, add IPA to make the overall weight 24 g, and stir for 1 hour to obtain solution B, set aside;
[0047] 3. Take TEOS 14.11 g, add H 2 O 2.82 ml, 1 M / mol HNO 3 2.83g, add IPA to make the overall mass 24g, magnetic stirrer for 1 h, obtain solution D, set aside;
[0048] 4. Mix solutions B, C, and D and stir them magnetically for 1 h, then add 10.65 g of Sr(NO 3 ) 2 , and continued magnetic stirring for 30 min after complete dissolution, then added 11.88 g Ca(NO 3 ) 2 • 4H 2 O, stirred for 30 min after complete dissolution to obtain solution F;
[0049] 5. Add solution A dropwise to the obtained homogeneous solution F, and magnetically stir for 30 min to obtain solution H;
[0050] 6. Add 1 M / L NH dropwise to Solution H 3 •H 2O until the pH = 11, stirring contin...
Embodiment 3
[0055] 1. Add 50 ml H to 16 g trichloroborazine 2 O, add 1 M / L NH dropwise 3 •H 2 O to PH=8, stirred at 45 °C for 24 h, A obtained solution A, set aside;
[0056] 2. Take 4.92 g ATSB, add 2.6 g EAA, add IPA to make the overall weight 24 g, stir magnetically for 1 hour, and obtain solution B, set aside;
[0057] 3. Take TEOS 14.11 g, add H 2 O 2.82 ml, 1 M / mol HNO 3 2.83g, add IPA to make the overall mass 24g, magnetic stirrer for 1 h, obtain solution D, set aside;
[0058] 4. Mix solutions B, C, and D and stir them magnetically for 1 h, then add 10.65 g of Sr(NO 3 ) 2 , after the complete dissolution, continue magnetic stirring for 30 min, add 11.88 g Ca(NO 3 ) 2 • 4H 2 O, after completely dissolving, stir for 30 min to obtain solution F;
[0059] 5. Add solution A dropwise to the obtained homogeneous solution F, and magnetically stir for 30 min to obtain solution H;
[0060] 6. Add 1 M / L NH dropwise to Solution H 3 •H 2 O until PH = 11, stirring continuously fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com