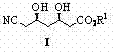
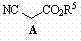Preparation method of (3R,5R)-3,5-dihydroxy-6-methyl cyan-caproate
A technology of cyanocaproate and dihydroxy, which is applied in the field of preparation of -3,5-dihydroxy-6-cyanocaproate, which can solve the problems of harsh reaction conditions, high requirements for equipment and labor protection, complicated operation, etc. problems, to achieve the effect of mild reaction conditions, good industrialization prospects, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
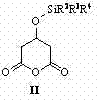
[0028] Step 1: Preparation of (R)-3-tert-butyldiphenylsilyloxy-5-methoxy-5-oxopentanoic acid ( )
[0029] 3--tert-butyldiphenylsilyloxycyclopentanoic anhydride ( )1.1 g (3 mmol, 1.0 eq), methanol 1.2 mL (30 mmol, 10 eq), quinine thiourea catalyst 0.3 mmol (0.1 eq) dissolved in 150 mL methyl tert-butyl ether, 0 o C was reacted for 24 hours, concentrated under reduced pressure, and purified by column chromatography to obtain colorless oily matter 3-tert-butyldiphenylsilyloxy-5-methoxy-5-oxopentanoic acid ( ) (1.16 g, 2.9 mmol), 97% yield. 1 H-NMR (400 MHz, CDCl 3 ) δ 1.04 (s, 9H), 2.55-2.68 (m, 4H), 3.57 (s, 3H), 4.50-4.56 (m, 1H), 7.36-7.47 (m, 6H), 7.68 (d, J = 6.8 Hz, 4H), 10.07 (br s, 1H) ppm. MS (ESI): 399 (M-H + );
[0030] Step 2: Preparation of (R)-dimethyl 2-cyano-3-oxo-5-tert-butyldiphenylsiloxypimelate ( )
[0031] 3-tert-butyldiphenylsilyloxy-5-methoxy-5-oxopentanoic acid ( ) 800 mg (2 mmol, 1.0 eq), 0.29 mL (3 mmol, 1.5 eq) of oxalyl chloride, ...
Embodiment 2
[0037] Step 1: Preparation of (R)-3-tert-butyldiphenylsilyloxy-5-benzyloxy-5-oxopentanoic acid ( )
[0038] 3-tert-butyldiphenylsilyloxycyclopentanoic anhydride ( ) 1.1 g (3 mmol, 1.0 eq), 3.1 mL (30 mmol, 10 eq) of benzyl alcohol, and 0.3 mmol (0.1 eq) of quinine sulfonamide catalyst were dissolved in 150 mL of tetrahydrofuran, and reacted at room temperature for 12 hours under a nitrogen atmosphere. Concentrate under reduced pressure, and purify by column chromatography to obtain colorless oil 3-tert-butyldiphenylsilyloxy-5-benzyloxy-5-oxopentanoic acid ( ) (1.4 g, 2.9 mmol), 97% yield. 1 H-NMR (400 MHz, CDCl 3 ) δ: 1.01 (s, 9H), 2.54-2.69 (m, 4H), 4.49-4.55 (m, 1H), 4.97 (d, J = 12.0 Hz, 1H), 5.03 (d, J = 12.0 Hz, 1H), 7.25-7.44 (m, 11H), 7.64-7.67 (m, 4H) ppm. MS (ESI): 477 (M+H + );
[0039] Step 2: Preparation of methylbenzyl (R)-2-cyano-3-oxo-5-tert-butyldiphenylsiloxypimelate ( )
[0040] 3-tert-butyldiphenylsilyloxy-5-benzyloxy-5-oxopentanoic acid...
Embodiment 3
[0046] Step 1: Preparation of (R)-3-tert-butyldimethylsilyloxy-5-methoxy-5-oxopentanoic acid ( )
[0047] 3-tert-butyldimethylsiloxycyclopentanoic anhydride ( ) 0.73 g (3 mmol, 1.0 eq), 1.2 mL (30 mmol, 10 eq) of methanol, and 0.3 mmol (0.1 eq) of quinine urea catalyst were dissolved in 150 mL of dichloromethane, and reacted at room temperature for 12 hours under a nitrogen atmosphere. Concentrate under reduced pressure, and purify by column chromatography to obtain 3-tert-butyldimethylsilyloxy-5-methoxy-5-oxopentanoic acid as a colorless oil ( ) (0.8 g, 2.9 mmol), yield 97%. 1 H-NMR (400 MHz, CDCl 3 ) δ 0.06 (s, 3H), 0.07 (s, 3H), 0.84 (s, 9H), 2.55-2.69 (m, 4H), 3.68 (s, 3H), 4.51-4.57 (m, 1H), 11.07 ( br s, 1H) ppm. MS (ESI): 275 (M-H + );
[0048] Step 2: Preparation of dimethyl (R)-2-cyano-3-oxo-5-tert-butyldimethylsiloxypimelate ( )
[0049] 3-tert-butyldimethylsilyloxy-5-methoxy-5-oxopentanoic acid ( ) 552 mg (2 mmol, 1.0 eq), 0.29 mL (3 mmol, 1.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com