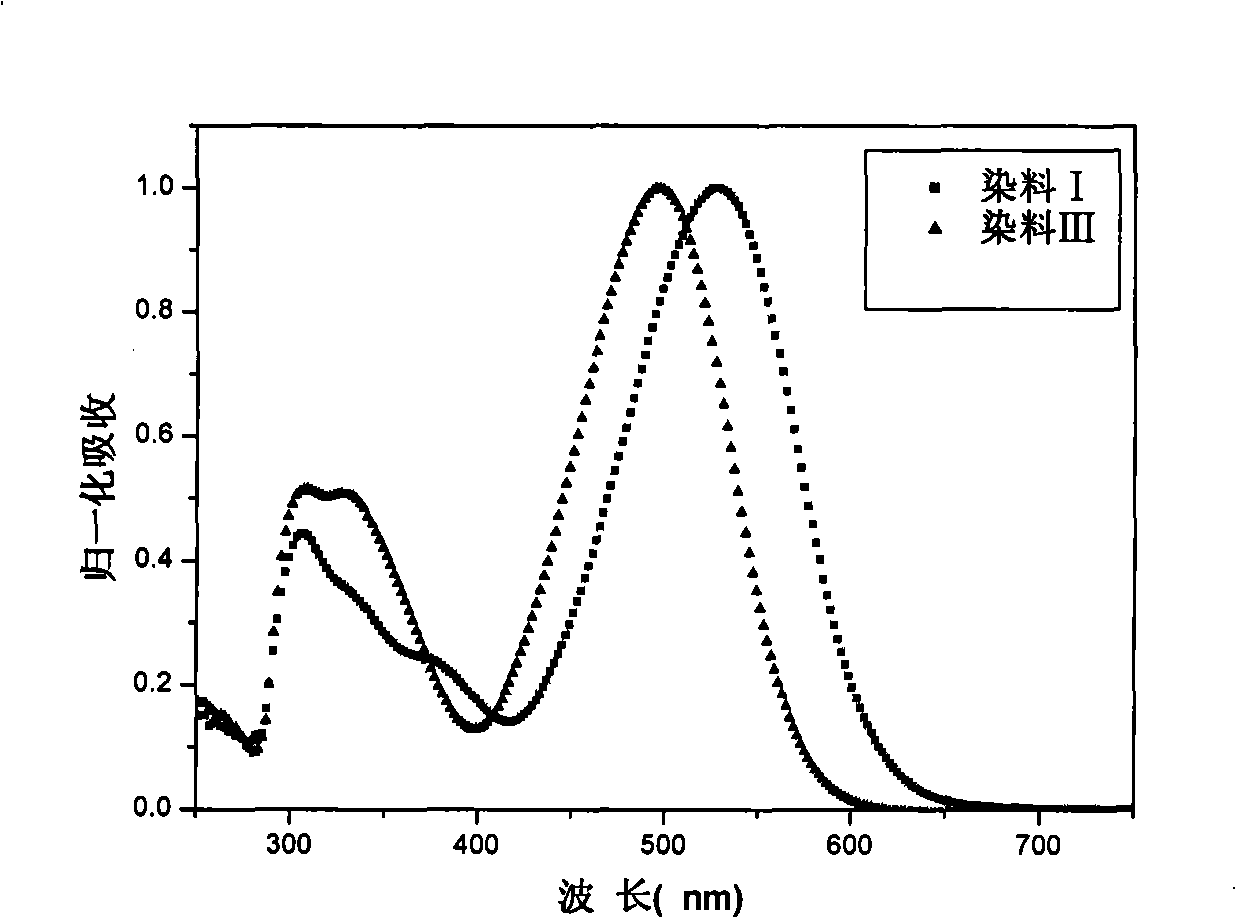
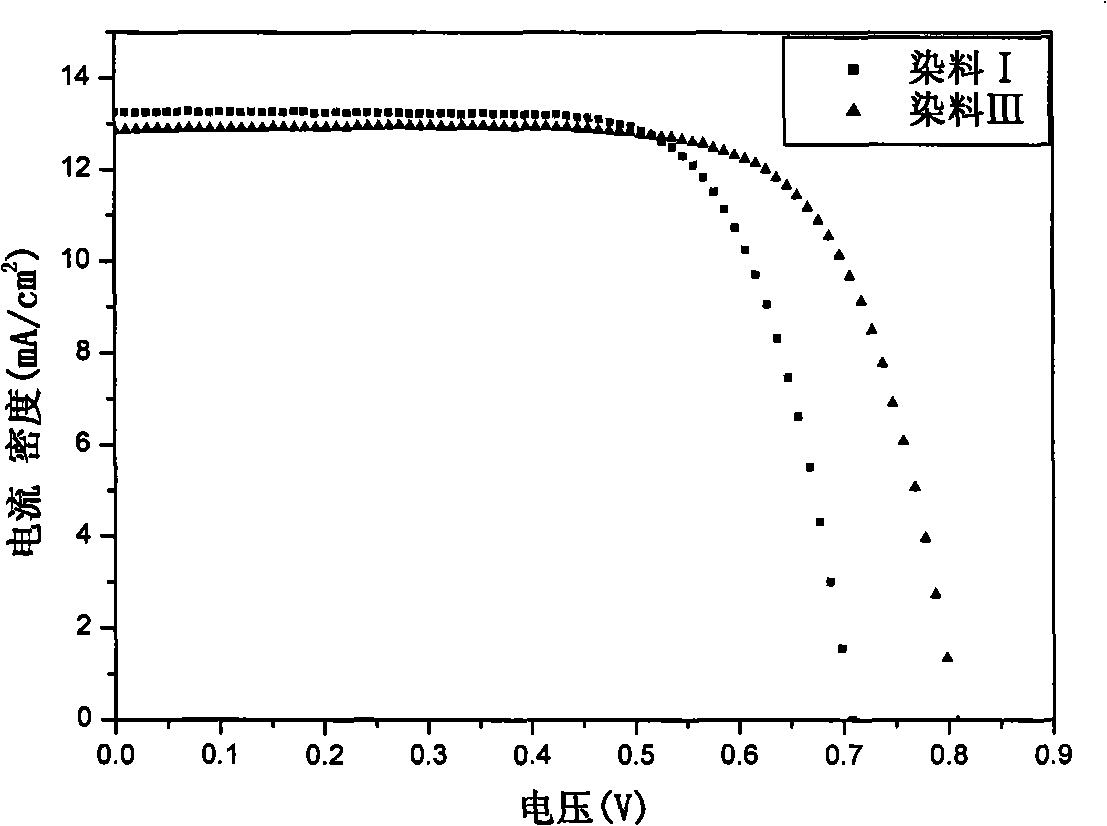
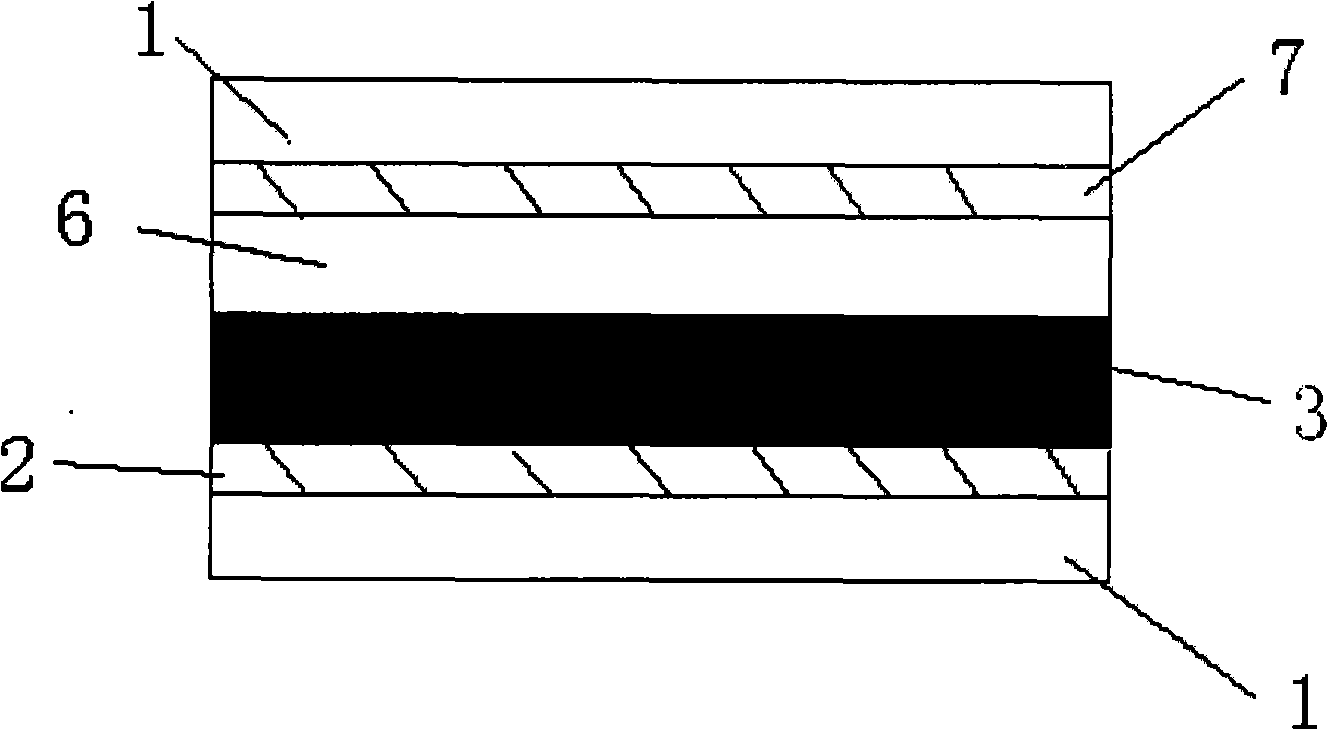
Organic dye with five-element heterocycle and derivative thereof as conjugate unit, and dye sensitization solar cell prepared thereby
A technology of organic dyes and derivatives, applied in the field of dye-sensitized solar cells, which can solve problems such as limiting practical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the synthetic route of dyestuff I is as follows:
[0071]
[0072] The specific synthesis method is:
[0073] Dissolve 5.24g of compound a-1, 5.5g of 2-selenophenetributyltin, and 0.7g of bistriphenylphosphinepalladium dichloride in 50mL of THF, reflux the reaction under argon for 12h, remove the solvent, and obtain the residue by column chromatography Compound b-1. Compound b-10.57g, phosphorus oxychloride 0.2mL, DMF and 10mL of chloroform were reacted at room temperature for 5h, diluted with chloroform, washed with saturated sodium bicarbonate solution and saturated brine successively, the solvent was removed, and the residue was subjected to column chromatography to obtain compound c -1. Compound c-10.5g, cyanoacetic acid 0.21g and piperidine 0.6mL were dissolved in 10mL chloroform, refluxed under argon for 4h, the solvent was removed, and dye I was obtained by column chromatography.
[0074] NMR data of dye I: 1 H NMR (400MHz, DMSO-d 6 )δ: 0.88...
Embodiment 2
[0076] Using 2,2'-diselenol-5-tributyltin instead of 2-selenophene tributyltin as the starting material,
[0077] Dye II was prepared according to the same experimental procedure as in Example 1.
[0078] NMR data of dye II: 1 H NMR (400MHz, DMSO-d 6 )δ: 0.88(t, 6H), 1.31(m, 8H), 1.40(m, 4H), 1.68(m, 4H), 3.94(t, 4H), 6.72(d, 2H), 6.91(d, 4H ), 7.04(d, 4H), 7.45(d, 2H), 7.48(d, 1H), 7.49(d, 1H), 7.66(d, 1H), 8.07(d, 1H), 8.40(s, 1H) .
Embodiment 3
[0080] Using 2-tributyltin furan instead of 2-tributyltin selenophene as the starting material, dye III was prepared according to the same experimental process as in Example 1.
[0081] NMR data of dye III: 1 H NMR (400MHz, DMSO-d 6 )δ: 0.88(t, 6H), 1.31(m, 8H), 1.42(m, 4H), 1.70(m, 4H), 3.94(t, 4H), 6.75(d, 2H), 6.92(d, 4H ), 7.08 (d, 4H), 7.11 (d, 1H), 7.51 (d, 1H), 7.70 (d, 2H), 7.982 (s, 1H), 13.48 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com