Isodiketopyrrolopyrrole dyes and application thereof
A technology of diketo rolopyrrole and diketo rolopyrrole group is applied in the field of organic dyes and dye-sensitized solar cells to achieve the effects of easy availability of raw materials, simple synthesis method and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
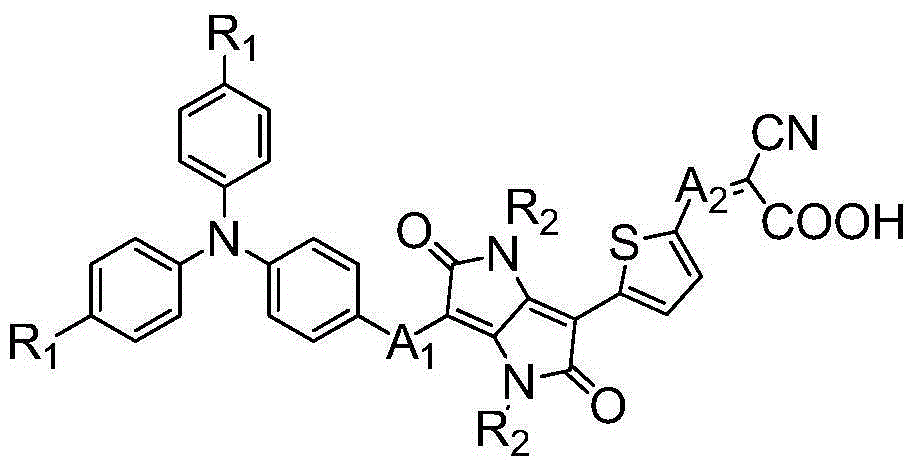
[0045] Synthesis of Dye isoDPP Containing Diketopyrrolopyrrole in π Bridge
[0046] (1) Synthesis of compound 2
[0047]
[0048] Add compound 1 (2.33g, 6.25mmol), CsCO 3 (6.11 g, 18.74 mmol) and 30 mL of dry DMF, the air in the apparatus was evacuated with a vacuum pump, and filled with Ar. The oil bath was heated to 60°C and stirred for 1 hour. A DMF (7 mL) solution of bromoisoctane (7.24 g, 37.50 mmol) was slowly injected into the above reaction solution with a disposable syringe. After the injection was completed, the temperature of the oil bath was raised to 120° C. to continue the reaction for 24 hours. After the reaction was completed, the temperature was lowered to room temperature, and the reaction liquid was stirred by adding dilute hydrochloric acid solution to adjust the pH value of the solution to be neutral. Extract with 3×50mL dichloromethane, combine the organic phases and wash with saturated brine, dry with anhydrous sodium sulfate, remove dichlorometha...
Embodiment 2
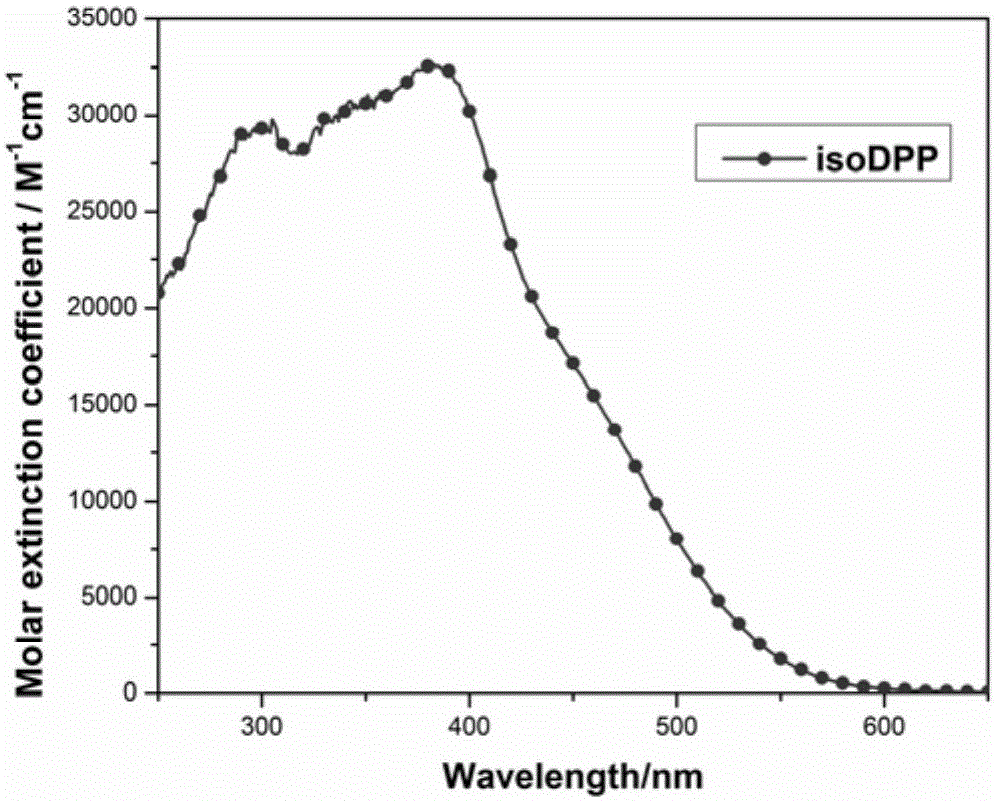
[0066] The dye that embodiment 1 obtains carries out ultraviolet-visible absorption spectrum test, and its ultraviolet-visible absorption spectrum is as figure 1 shown. Solvent: 4-tert-butanol / acetonitrile mixed solution (v / v=1 / 1)
[0067] Concentration: 2×10 -5 m
[0068] Temperature: room temperature
[0069] Instrument: ShimadzuUV-2450 UV-Vis spectrophotometer
[0070] from figure 1 It can be seen that in the 4-tert-butanol / acetonitrile mixed solution (v / v=1 / 1), unlike most pure organic dyes, the dye isoDPP only exhibits one absorption peak, but presents a wider coverage. Over 10,000M in the 400–490nm range -1 cm -1 , which indicates that the dye has good light-harvesting ability.
Embodiment 3
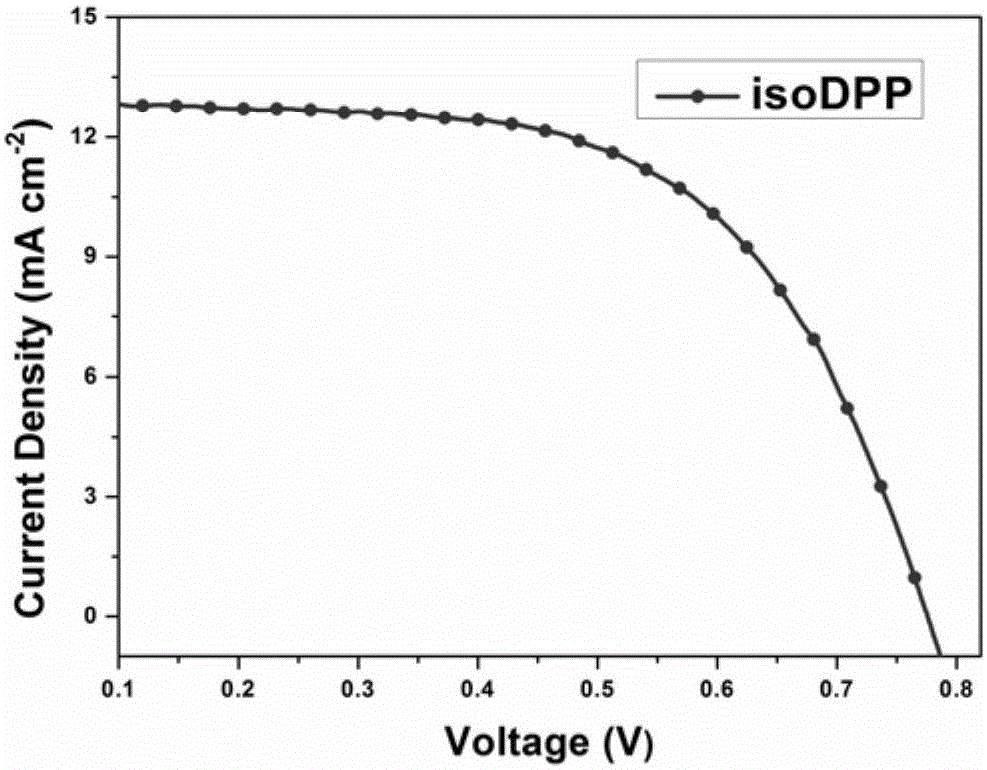
[0072] The making of dye-sensitized solar cell among the present invention is as follows:
[0073] (1) Pretreatment of conductive glass (FTO): ultrasonically clean the cut FTO (2×5 cm), and rinse it with deionized water 4 times. Then soak in KOH saturated ethanol solution for 24h. Then use deionized water, acetone, deionized water and ethanol to ultrasonically clean, dry and store for later use;
[0074] (2) Preparation of photoanode: at room temperature, 15mLTi(OBu) 2 Add 50 mL of acetic acid and deionized water to the mixed solution with 20 mL of EtOH under vigorous stirring and continue to stir for 1 h. The mixture was transferred into an autoclave lined with Teflon (polytetrafluoroethylene), treated at 230° C. for 12 h, and then cooled to room temperature naturally. The resulting suspension was filtered, washed with deionized water and ethanol four times (, and dried in an oven at 50°C for 6 hours, until dry to obtain TiO 2 nanocrystalline particles. To the prepared T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com