Three-dimensionally prolonged conjugated chain phenothiazine dyes and application to dye-sensitized solar cells thereof
A dye-sensitized, solar cell technology, applied to three-way extended conjugated chain phenothiazine dyes and its application in dye-sensitized solar cells, can solve the limitations of large-scale practical application, separation of ruthenium polypyridine complexes Difficulty in purification, difficulty in reducing the cost of DSSCs, etc., to achieve the effect of improving photoelectric conversion efficiency, cheap raw materials, and enhancing absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
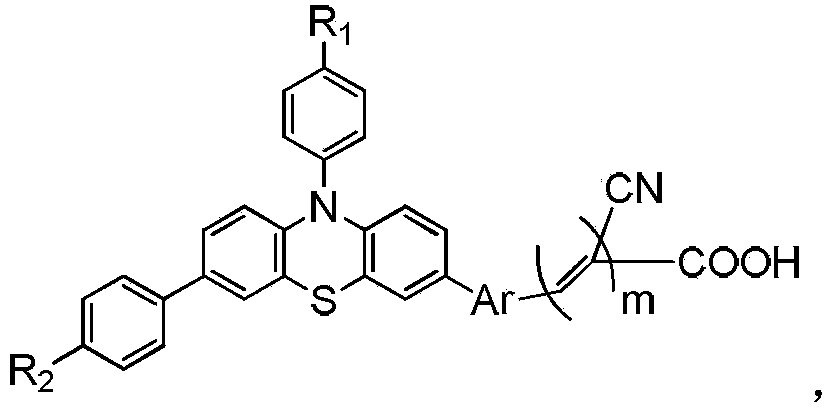
[0039] Synthesis of three-way extended conjugated chain phenothiazine dye PPFA
[0040] The following reactions were all carried out in a dry environment under nitrogen protection.
[0041] (1) Synthesis of compound 2
[0042]
[0043] 0.81 g (2 mmol) of compound 1 was dissolved in 150 ml of chloroform and kept at 0°C. Then 20ml of chloroform solution dissolved with 0.22ml (4mmol) of liquid bromine was added dropwise into the flask while keeping the temperature at around 0°C. The reaction was stirred for 8h. Stirring was continued for 1 h after adding a dilution of NaOH. Adding salt water is conducive to stratification. Use 200ml CH 2 Cl 2 Extract and wash twice with 200ml of water. After drying, filtering, and removing the solvent, the crude product was separated and purified by column chromatography (dichloromethane as mobile phase) to obtain a bright green product with a yield of 92.5%. Melting point: 107–108°C. NMR 1 H NMR (CDCl 3 ,400MHz,δ,ppm):7.25–7.22(m,2...
Embodiment 2
[0057] Synthesis of three-way extended conjugated chain phenothiazine dye PPTA
[0058] All reactions were carried out in a dry environment under nitrogen protection
[0059]
[0060] 2.25g (4mmol) compound 2 and 2.21g (16mmol) K 2 CO 3 and Pd(PPh 3 ) 4 (10mole%) was added to 40ml of DMF, stirred and reacted at 40°C for 0.5h under the protection of argon. Then, 0.49 g (3.5 mmol) of 5-formyl-2-thiopheneboronic acid dissolved in 5 ml of DMF was slowly added dropwise under stirring, and after the addition was completed, the temperature was raised to 80° C. for 48 h. Cool after the reaction is over, pour the reaction solution into 200ml water, and use CH 2 Cl 2 extraction. The organic layer was washed several times with water to completely remove DMF. After drying and concentration, the crude product was separated and purified by column chromatography (petroleum ether and dichloromethane (1:1) as mobile phase) to obtain 0.87 g of orange product with a yield of 36.9%. M...
Embodiment 3
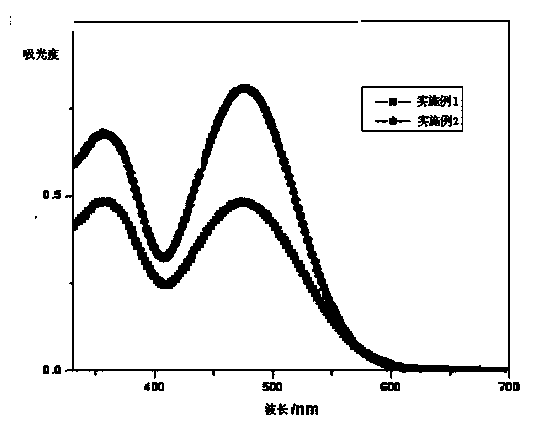
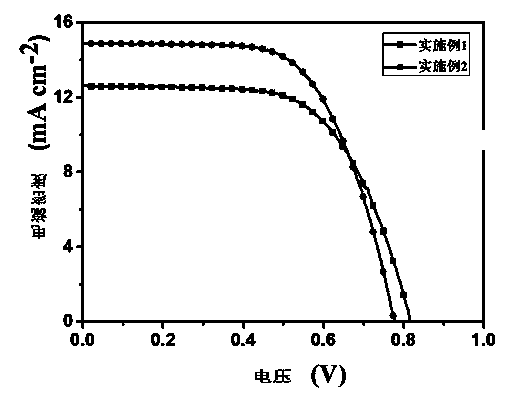
[0071] The ultraviolet-visible absorption spectrum / fluorescence spectrum test of embodiment 1 and embodiment 2 dyestuff, ultraviolet-visible absorption spectrum and fluorescence emission spectrogram are respectively figure 1 , figure 2 shown.
[0072] Solvent: tetrahydrofuran / dichloromethane (1:1)
[0073] Concentration: 2×10 -5 m
[0074] Temperature: room temperature
[0075] Instruments: Shimadzu UV-2450 UV-visible scenery photometer, Hitachi F-4500 fluorescence spectrometer
[0076] The resulting data are summarized in Table 1
[0077] dye Maximum UV / Vis Absorption Wavelength (nm) Maximum fluorescence emission wavelength (nm) Example 1 358,473 596 Example 2 359,476 571
[0078]The maximum ultraviolet / visible absorption wavelength and the maximum fluorescence emission wavelength (nm) data of dyestuff in table 1 embodiment 1 and 2
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com