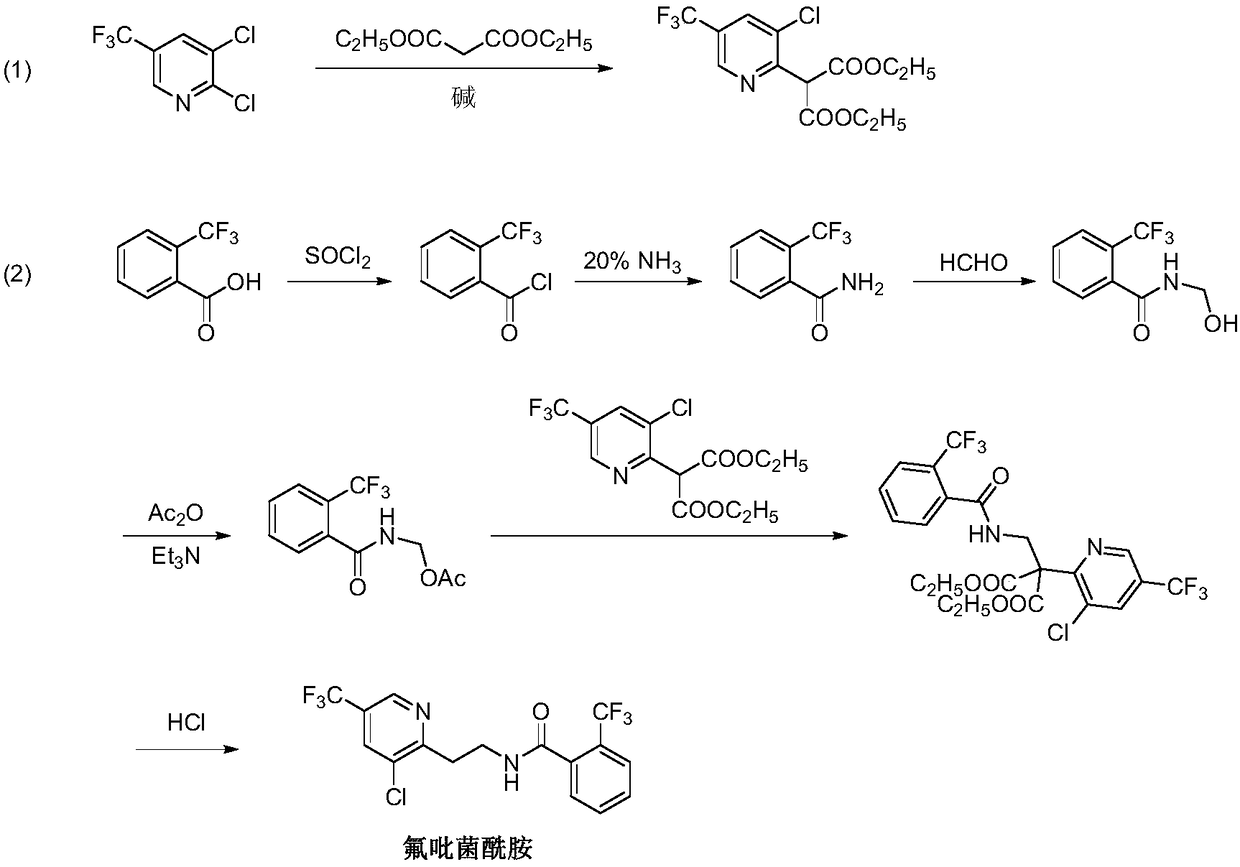
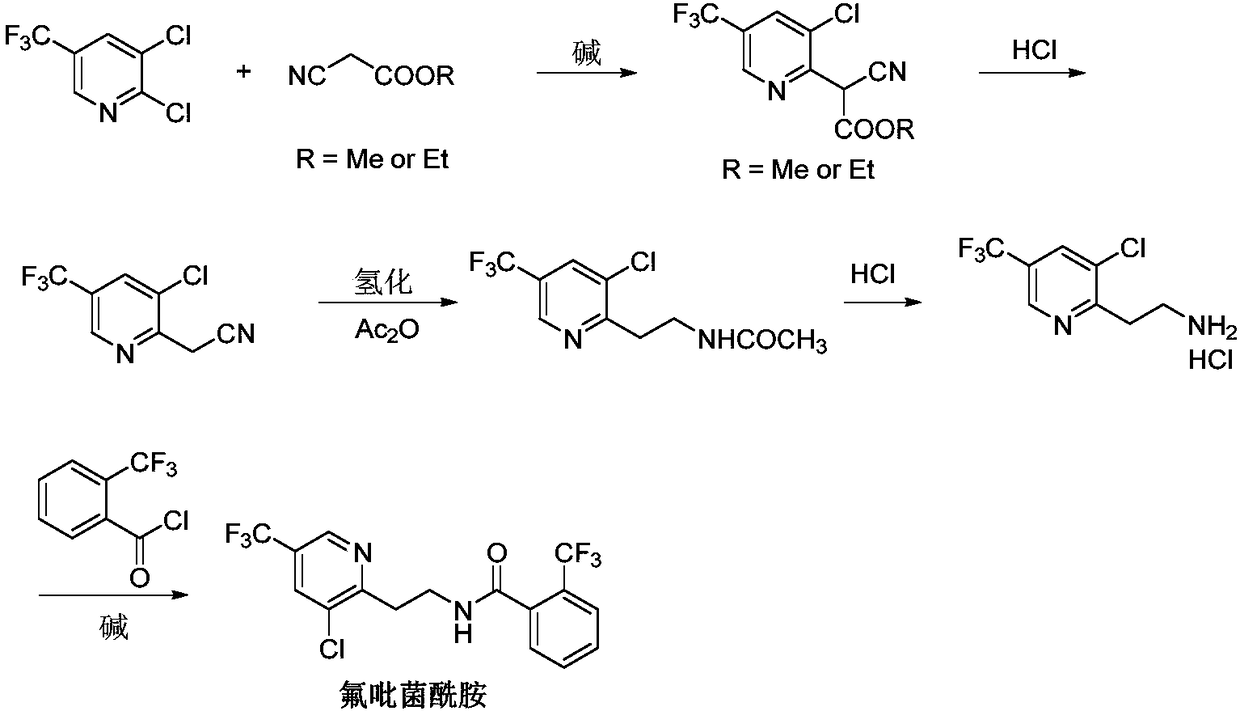
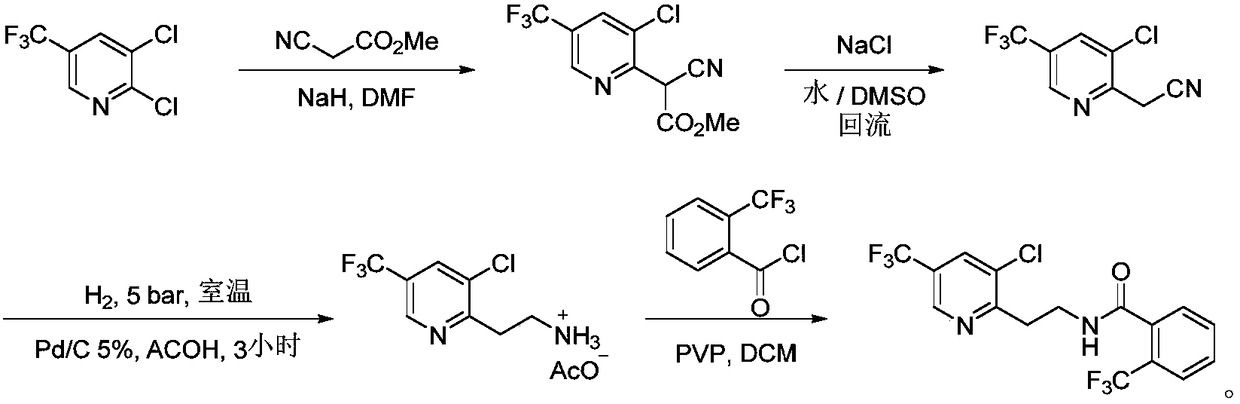
Fluopyram preparation method
A technology of fluopyram and trifluoromethylpyridine, which is applied in the field of preparation of fluopyram, can solve the problems of excessive three wastes and low reduction reaction yield, and achieve the effect of short steps and avoiding deprotection steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of fluopyram of the present embodiment has the following steps:
[0038] 2,3-Dichloro-5-trifluoromethylpyridine (10.0 g, 46.5 mmol), potassium carbonate (7.7 g, 55.8 mmol) were added to N,N-dimethylformamide (50 mL). Ethyl cyanoacetate (6.3 g, 55.8 mmol) was added dropwise to the above mixture at room temperature. After the dropwise addition was complete, the reaction was reacted at 70° C. for 3 hours. After the reaction was completed, it was cooled to room temperature. The pH was adjusted to 2-3 with hydrochloric acid, and the reaction solution was stirred at 140°C for 16 hours. The reaction solution was cooled to room temperature, adjusted to pH 8-9 with 30% potassium hydroxide solution, and extracted with ethyl acetate. The organic phase was concentrated and then distilled under reduced pressure to obtain 3‐chloro‐5‐(trifluoromethyl)‐2‐acetonitrile pyridine (8.63 g) as a yellow oily liquid with a yield of 81% and a purity of 96%.
[0039] A...
Embodiment 2
[0041] The preparation method of fluopyram of the present embodiment has the following steps:
[0042] 2,3-Dichloro-5-trifluoromethylpyridine (10.0 g, 46.5 mmol), potassium hydroxide (2.6 g, 46.5 mmol) were added to dimethylsulfoxide (50 mL). Ethyl cyanoacetate (5.25 g, 46.5 mmol) was added dropwise to the above mixture at room temperature. After the dropwise addition was complete, the reaction was reacted at 70° C. for 3 hours. After the reaction was completed, it was cooled to room temperature. The pH was adjusted to 2-3 with hydrochloric acid, and the reaction solution was stirred at 160° C. for 13 hours. The reaction solution was cooled to room temperature, adjusted to pH 8-9 with 30% potassium hydroxide solution, and extracted with ethyl acetate. The organic phase was concentrated and then distilled under reduced pressure to obtain 3-chloro-5-(trifluoromethyl)-2-acetonitrile pyridine (8.5 g) as a yellow oily liquid with a yield of 79% and a purity of 95%.
[0043] Add...
Embodiment 3
[0045]The preparation method of fluopyram of the present embodiment has the following steps:
[0046] 2,3-Dichloro-5-trifluoromethylpyridine (10.0 g, 46.5 mmol), potassium carbonate (9.6 g, 70.0 mmol) were added to dimethylsulfoxide (70 mL). Methyl cyanoacetate (6.93 g, 70.0 mmol) was added dropwise to the above mixture at room temperature. After the dropwise addition was complete, the reaction was carried out at 80° C. for 2 hours. After the reaction was completed, it was cooled to room temperature. The pH was adjusted to 2-3 with hydrochloric acid, and the reaction solution was stirred at 160° C. for 14 hours. The reaction solution was cooled to room temperature, adjusted to pH 8-9 with 30% potassium hydroxide solution, and extracted with ethyl acetate. The organic phase was concentrated and distilled under reduced pressure to obtain 3‐chloro‐5‐(trifluoromethyl)‐2‐acetonitrile pyridine (8.84 g), a yellow oily liquid with a yield of 83% and a purity of 96%.
[0047] Add 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com