Double-strand phenothiazine dye with benzotriazole led into Pi bridge and application thereof in preparation of dye-sensitized solar cells
A benzotriazole and solar cell technology, applied in thiazine dyes, organic dyes, photovoltaic power generation, etc., can solve problems such as difficulty in cost reduction, limitation of large-scale practical application, difficulty in separation and purification of ruthenium polypyridine complexes, etc. To achieve the effect of increasing adsorption capacity, improving photoelectric conversion efficiency, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
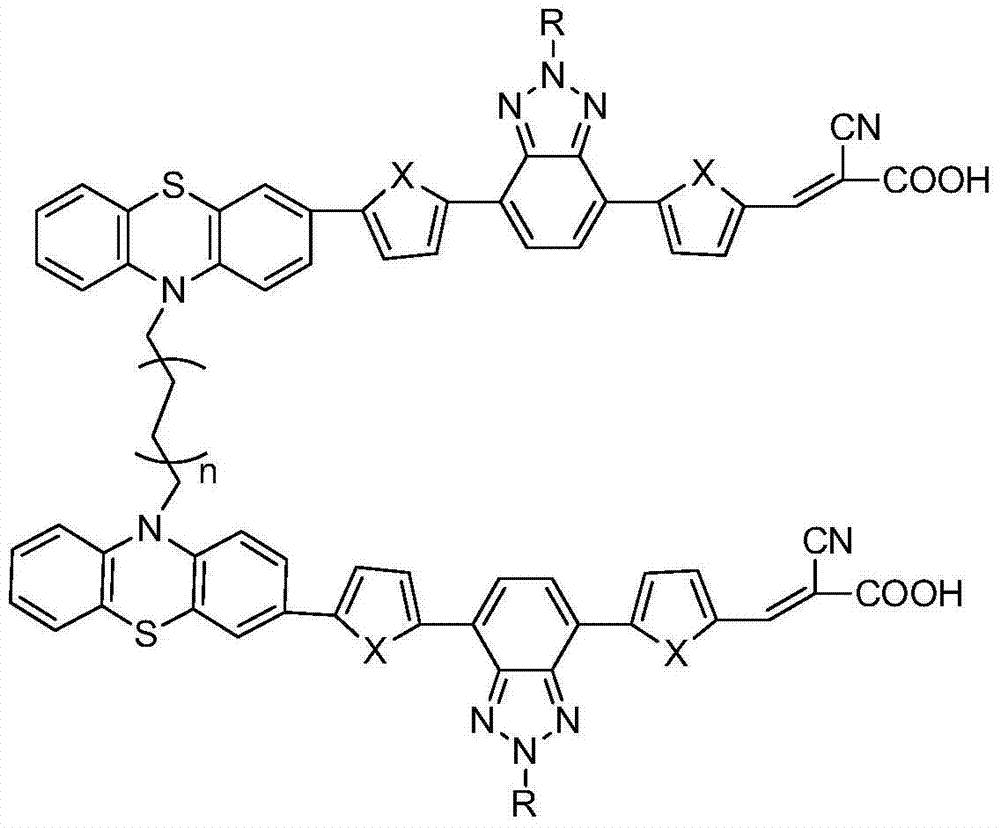
[0040] Synthesis of double chain phenothiazine dye HZ containing benzotriazole in π bridge
[0041] (1) Synthesis of compound 2
[0042]
[0043] Under the protection of argon, with 50mLTHF as solvent, add 1.43g (3.67mmol) compound 1, 11mL2M K 2 CO 3 solution and 1.127g (8.81mmol) 2-thiophene boronic acid, and then add 211mg of Pd (PPh 3 ) 4 Be a catalyst. Then the temperature was raised to 70°C for 24h. After cooling to room temperature, the reaction solution was poured into water, extracted with 200 mL of DCM, and washed twice with 200 mL of water. After drying, filtering, and removing the solvent, the crude product was separated and purified by column chromatography, using ethyl acetate:petroleum ether (1:20) as the mobile phase, to obtain a light yellow product with a yield of 84%. Melting point: 74-76°C. NMR: 1 H NMR (400MHz, CDCl 3 )δ8.09-8.08(m,2H),7.61(s,2H),7.37-7.36(m,2H),7.19-7.17(m,2H),4.80(t,J=7.2Hz,2H),2.22 -2.15(m,2H),1.45-1.34(m,2H),1.33-1.27(m,6H)...
Embodiment 2
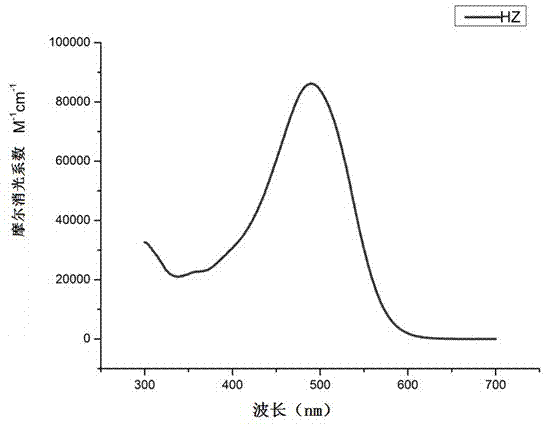
[0060] To the ultraviolet-visible absorption spectrum test of embodiment 1, ultraviolet-visible absorption spectrum is as figure 1 shown.
[0061] Solvent: tetrahydrofuran / dichloromethane (1:1)
[0062] Concentration: 2×10 -5 m
[0063] Temperature: room temperature
[0064] Instrument: Shimadzu UV-2450 UV-Vis spectrophotometer
[0065] The resulting data are summarized in Table 1
[0066] The ultraviolet-visible spectral data of table 1 embodiment 1 dyestuff
[0067] dye
[0068] From figure 1 It can be seen from Table 1 that the maximum absorption wavelength of the dye HZ in the mixed solvent of tetrahydrofuran / dichloromethane (1:1) is 489nm, and this peak is caused by the intramolecular charge transfer (ICT) of the dye. The corresponding molar extinction coefficient of the dye HZ at the maximum absorption wavelength reaches 86208M -1 cm -1 , so that the data is much higher than that of common pure organic dyes; this indicates that this series of dyes has ...
Embodiment 3
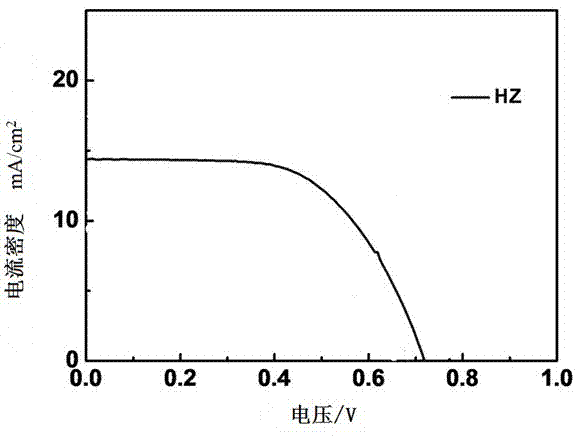
[0070] The making of dye-sensitized solar cell among the present invention is as follows:
[0071] (1) Pretreatment of conductive glass (FTO): The conductive glass is fully cleaned with ultrasonic waves in degreasing agent, absolute ethanol and deionized water in sequence, and then dried for later use;
[0072] (2) TiO 2 Preparation of nanocrystalline particles and their slurry: at room temperature, 10mL Ti(OBu) 4 After stirring the mixture with 20mL of EtOH for 10 minutes, add 18mL of acetic acid and 50mL of deionized water to the above solution under vigorous stirring and keep stirring for 1h, then transfer the mixture into an autoclave at 230°C for 12h, and naturally cool to room temperature , filter the resulting suspension, wash with deionized water and ethanol several times respectively, and dry in an oven at 50°C for 6h to dry to obtain TiO with a particle size of about 18-20nm. 2 Nanocrystalline particles;
[0073] (3) Take TiO 2 Add 1.0g of nanocrystalline particl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Molar extinction coefficient | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com