Organic dye containing calixarene derivative and preparation method and application thereof
A technology of organic dyes and derivatives, applied in the field of organic dyes, to achieve the effect of improving light harvesting ability, increasing service life, and inhibiting molecular aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
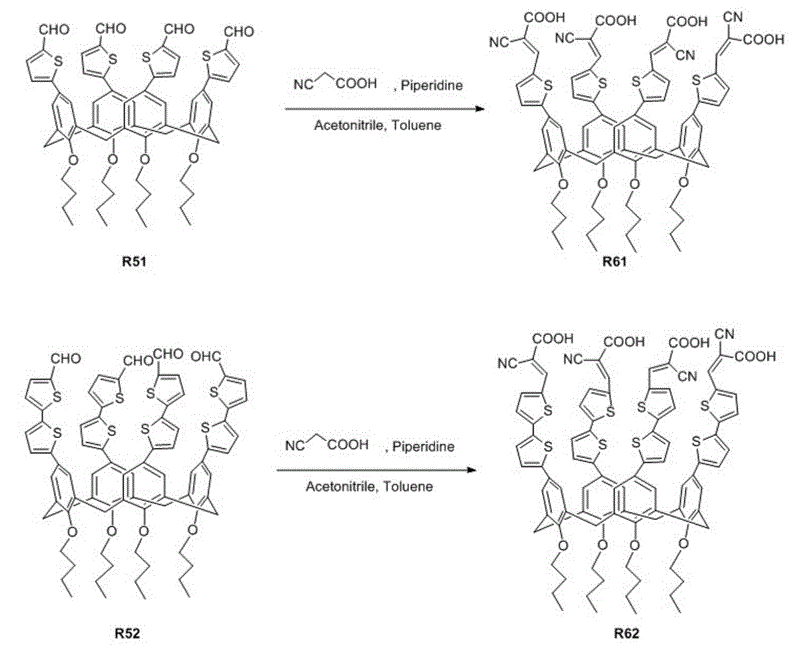
[0043] Organic dyes containing calixarene derivatives. The dye uses calixarene derivatives as electron donors, cyanoacetic acid as electron acceptors, and thiophene and its derivatives as conjugated bridging groups. The organic dyes in this embodiment are referred to as R61. Its structural formula is as follows:
[0044]
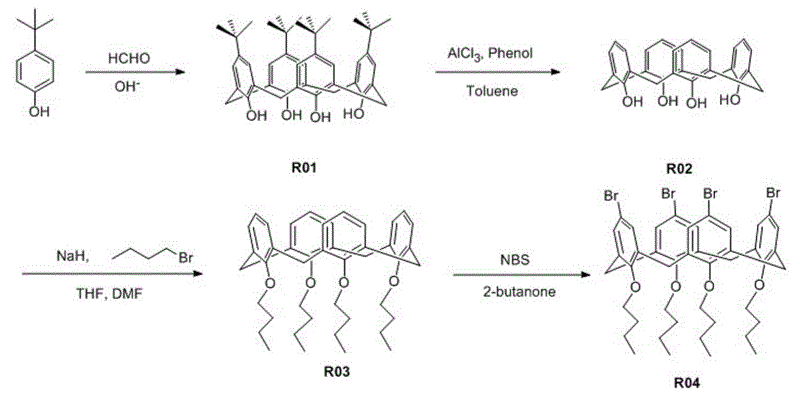
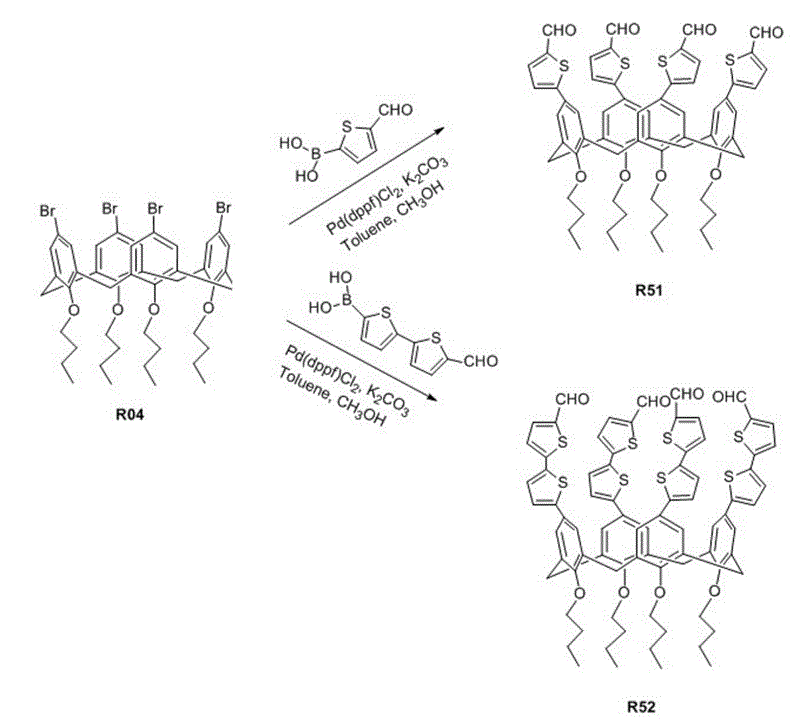
[0045] See the synthetic route of organic dye R61 Figure 1 ~ Figure 3 As shown, the preparation process is as follows:
[0046] 1) Synthesis of p-tert-butyl calix[4] arene (R01)
[0047] Add 0.1 mol of p-tert-butylphenol, 3.8 mmol of sodium hydroxide and 20 mL of 37% formaldehyde solution into a 250 mL three-necked flask equipped with an oil-water separator, condenser and nitrogen protection device. Stir mechanically under nitrogen protection until dissolved ; Slowly heat to reflux state, evaporate most of the water in the oil-water separator, remove the water separator; keep stirring and reflux for 2 h at 110~120℃, then cool to room temperature, the reactant is ...
Embodiment 2
[0064] Organic dyes containing calixarene derivatives. The dye uses calixarene derivatives as electron donors, cyanoacetic acid as electron acceptors, and thiophene and its derivatives as conjugated bridging groups. The organic dyes in this embodiment are referred to as R62. Its structural formula is as follows:
[0065]
[0066] See the synthetic route of organic dye R62 Figure 1 ~ Figure 3 As shown, the preparation process is as follows:
[0067] 1) The synthesis method of compound R01, R02, R03, R04 is the same as in Example 1;
[0068] 2) Synthesis of 5,11,17,23-tetra(5'-formyldithienyl)-25,26,27,28-tetrabutoxycalix[4]arene (R52)
[0069] Add 0.2 mmol R04, 1 mmol 5'-formyl-2,2'-bithiophene-5-boronic acid, 3 mmol anhydrous potassium carbonate, 4 ml toluene and 1 ml methanol into the Schlenk bottle, stir at room temperature for about 15 minutes, and then add 0.08 mmol Pd(dppf)Cl 2 , Mix well, warm up to 100°C, react for 20 h; extract the reaction product with dichloromethane, wash...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com