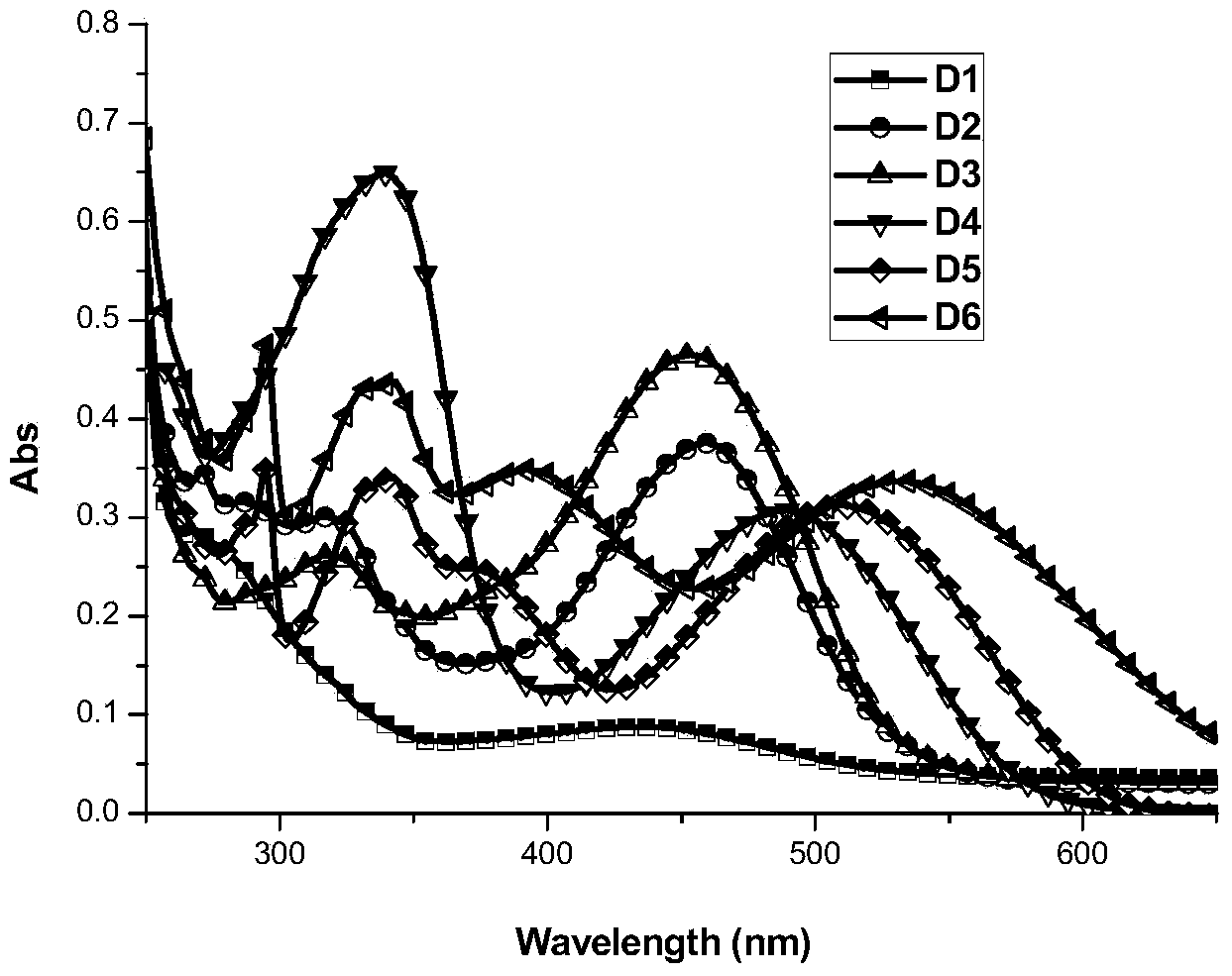
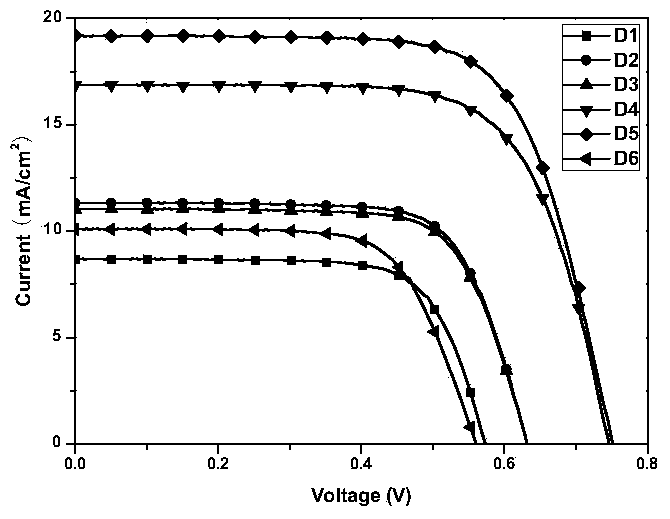
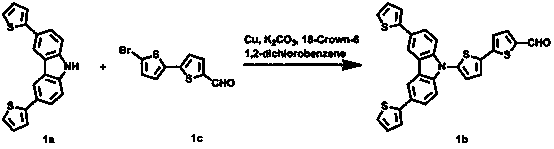Tree-based organic dyestuff based on carbazole or triphenylamine derivatives and application thereof in preparation of dye-sensitized solar cell
A technology of organic dyes and triphenylamine, applied in the field of dendritic organic dyes, can solve the problems of cumbersome purification and expensive rare metals, achieve high conversion efficiency, inhibit molecular aggregation, and prolong conjugation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1. Synthesis of dye D1
[0054] 1. Compound 1a [3,6-di(thiophen-2-yl)-9H-carbazole]
[0055]
[0056] Take a 100 mL schlenk bottle, vacuumize and nitrogen three times repeatedly to form an anhydrous and oxygen-free system. While maintaining nitrogen gas flow, the I 2 BocCz (3 mmol), 2-boronic acid thiophene (6.6 mmol), tetrakis (triphenylphosphine) palladium (0.12 mmol), K 3 PO 4(12 mmol) into the reactor. After removing water and oxygen from DMF, add 20 mL with a syringe as a reaction solvent. The oil bath was heated to 100 °C and stirred overnight (16 h), the reaction solution was cooled to room temperature, extracted with dichloromethane and water, and the organic phase was retained. After the solid was obtained by rotary evaporation, it was dissolved in 10 mL of dichloromethane, 5 mL of trifluoroacetic acid (TFA) and 1.5 mL of anisole were added. After reacting for 2 h at room temperature, extract with water and keep the organic phase. The crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com