Asymmetric synthesis method of (+)-tanshinol
A synthetic method and asymmetric technology, applied in the field of medicine and chemical industry, can solve the problems of unseen chemical synthesis of natural (+)-danshensu, chiral resolution of refractory racemic danshensu, etc., and achieve high optical purity and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
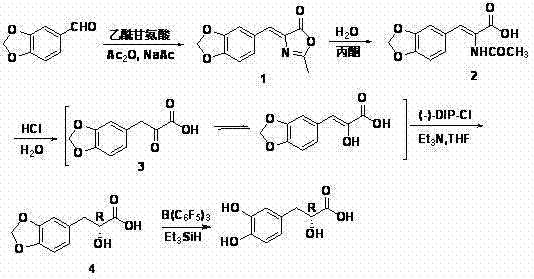
[0016] Example 1 Synthesis of 2-methyl-4-(3,4-methylenedioxybenzylidene)oxazolone (1)
[0017] Add 6.00 g (40 mmol) of piperonal, 5.76 g (50 mmol) of acetylglycine, 4.16 g (50 mmol) of anhydrous sodium acetate and 20 mL of acetic anhydride into a 250 mL three-necked flask, stir and react in a water bath at 80°C for 3 hours, then raise the temperature Continue the reaction at 100°C for 1 hour, stir and cool until the reaction mixture solidifies, add 30 mL of ice water and stir vigorously, a large number of yellow crystals precipitate, filter with suction, wash with ice water, and dry to obtain 6.80 g of bright yellow crystals, m.p.182.5°C-183.0 °C (literature value 178°C-179°C) (Wong, H. Synthesis, 1992, 793-797), yield: 75%. 1 H NMR (300 MHz, DMSO): δ 2.38 (s, 3H), 6.13 (s, 2H), 7.06(d, J = 8.1 Hz, 1H), 7.16 (s, 1H), 7.66(d, J = 8.1 Hz, 1H), 7.91 (s, 1H).
Embodiment 2
[0018] Example 2 Synthesis α -Acetylamino- β -(3,4-methylenedioxyphenyl)acrylic acid (2)
[0019] Add 4.62 g (20 mmol) of 2-methyl-4-(3,4-methylenedioxybenzylidene) oxazolone, 40 mL of water, 80 mL of acetone and 3.0 mL of concentrated hydrochloric acid into a 250 mL one-necked flask , reflux for 3h. Activated carbon decolorization, suction filtration while hot, after the filtrate was placed at room temperature, it was cooled in a refrigerator to complete the crystallization. Suction filtration, washing with ice water, and drying gave 3.06 g of loose beige crystalline powder, m.p. Rate: 75.5%. 1 H NMR (300 MHz, DMSO): δ 1.98 (s, 3H), 6.06 (s, 2H), 6.95(d, J = 8.0 Hz, 1H), 7.11~7.22 (m, 3H), 9.32 (s, 1H).
Embodiment 3
[0020] Example 3 Synthesis β -(3,4-methylenedioxyphenyl)pyruvate (3)
[0021] Add to a 250 mL one-necked flask α -Acetylamino- β -(3,4-methylenedioxyphenyl)acrylic acid 4.98 g (20 mmol) and 50 mL 3 mol L -1 Hydrochloric acid solution, reflux for 10 h. Decolorize with activated carbon, filter with suction, concentrate the filtrate until crystals are precipitated, and put it in the refrigerator to cool to complete the crystallization. Suction filtration, washing with ice water, and drying yielded 3.40 g of loose crystals, m.p. 1 H NMR (500 MHz, DMSO): δ 6.01(s, 2H), 6.77 (s, 1H), 6.90(d, J = 8.1 Hz, 1H), 7.14(d, J = 8.0 Hz, 1H), 7.44 (s, 1H), 9.08 (s, 1H), 13.08 (s, 1H); 13 C NMR (125 MHz, DMSO): δ100.99, 108.29, 108.89, 109.86, 123.83, 129.20, 140.36, 146.37, 147.21, 166.38.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com