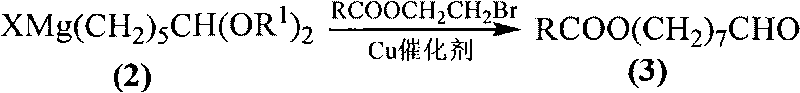Synthetic method of effective component-royaljelly acid of royal jelly
A technology of effective components and synthesis methods, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low coupling reaction yield, limited use, cumbersome synthesis routes, etc., to avoid difficulties in product purification , high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 8-acetoxyoctanal:
[0037] Add bromoethanol acetate (70.3mmol), N-methyl-2-pyrrolidone (27.88g, 0.281 mol, 4 equivalents based on bromoethanol acetate), 30 mL of anhydrous tetrahydrofuran, then the temperature of the system was lowered to -10 ° C, cuprous iodide (2.11 mmol, 3 equiv%), and then 1,1-ethylenedioxy -6-Hexylmagnesium bromide (1.1 equivalents based on bromoethanol acetate) was transferred to a constant pressure dropping funnel.
[0038] Slowly add 1,1-ethylenedioxy-6-hexylmagnesium bromide dropwise to the system, stir vigorously, keep the temperature of the system at -10°C, and the dropwise addition is completed in about 1 hour. After the dropwise addition, the system was kept at -10°C for 0.5h. After the reaction was completed, 10 mL of saturated ammonium chloride aqueous solution was added dropwise to the system, and then 2M hydrochloric acid was added to the system to adjust the pH to 4-5. The system continued to stir for 24 hours. Most o...
Embodiment 2
[0042] Preparation of 8-propionyloxyoctanal
[0043]Add bromoethanol propionate (70.3mmol), N-methyl-2-pyrrolidone (27.88g, 0.281 mol, 4 equivalents based on bromoethanol propionate), 30 mL of anhydrous tetrahydrofuran, then the temperature of the system was lowered to -50 °C, cuprous chloride (4.22 mmol, 6 equiv%), and then 1,1-dimethoxy -6-hexylmagnesium chloride (1.1 equivalents based on bromoethanol propionate) was transferred to a constant pressure dropping funnel. Slowly add 1,1-dimethoxy-6-hexylmagnesium chloride dropwise to the system, stir vigorously, keep the temperature of the system at -50°C, and the dropwise addition is completed in about 1 hour. After the dropwise addition, the system was kept at -50° C. for 0.5 h. After the reaction was completed, 10 mL of saturated ammonium chloride aqueous solution was added dropwise to the system, and then 10% phosphoric acid was added to the system to adjust the pH value to 4-5. The system continued to stir for 24 hours. ...
Embodiment 3
[0046] Preparation of 8-Acetoxyoctanal
[0047] Add bromoethanol propionate (70.3mmol), N-methyl-2-pyrrolidone (0.352mol, based on 5 equivalents of bromoethanol propionate), 30mL of anhydrous tetrahydrofuran, then the temperature of the system was raised to 50°C, Li 2 CuCl 4 (4.22mmol, 6mol%), and then its 1,1-dimethoxy-6-hexylmagnesium bromide (1.1 equivalents based on bromoethanol propionate) was transferred to a constant pressure dropping funnel. Slowly add 1,1-diethoxy-6-hexylmagnesium bromide dropwise to the system, stir vigorously, keep the temperature of the system at 50°C, and the dropwise addition is completed in about 1 hour. After the dropwise addition, the system was kept at 50° C. for 0.5 h. After the reaction was completed, 10 mL of saturated ammonium chloride aqueous solution was added dropwise to the system, and then 0.5 g of p-toluenesulfonic acid was added to the system. The system continued to stir for 24 hours. Most of the tetrahydrofuran was then disti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com