Novel 2,6-site-substituted BODIPY organic dye sensitizer and preparation method therefor
A technology of organic dyes and sensitizers, which is applied in the field of dye-sensitized solar cell materials, can solve the problems of limited resources and high prices, and achieve the effects of simple product purification, low production costs, and increased yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] (1) Synthesis of Dye1:
[0106] Intermediate 14 (180mg, 0.3mmol), cyanoacetic acid (50mg, 0.6mmol), acetonitrile (20mL), chloroform (20mL) and piperidine (0.2mL) were sequentially added to a 100mL three-necked flask, under argon protection, Reflux at 80°C for 24h. After cooling to room temperature, the reaction solution was poured into 50 mL of dichloromethane for dilution, washed with water 3 times (3×50 mL), and the organic phase was dried with anhydrous MgSO4. The solvent was removed by rotary evaporation, and the crude product was subjected to silica gel column chromatography (dichloromethane: methanol = 10:1) to obtain 156 mg of dark red solid powder with a yield of 80%. 1 HNMR(400MHz, CDCl 3 )δ10.15(s,1H), 8.17(d,J=7.4Hz,2H), 7.70(d,J=7.2Hz,2H), 7.51(s,2H),7.46(d,J=6.5Hz, 4H), 7.32(s, 2H), 3.17(t, 2H), 2.83(s, 3H), 2.81(s, 3H), 2.63(s, 3H), 2.50(s, 3H), 1.74(s, 2H) ), 1.54(d,J=4.6Hz,2H),1.43(d,J=6.5Hz,2H),0.96(t,3H). 13 CNMR(101MHz, CDCl 3 )δ:186.34,158.42,149.58,1...
Embodiment 2
[0108] (2) Synthesis of Dye2:
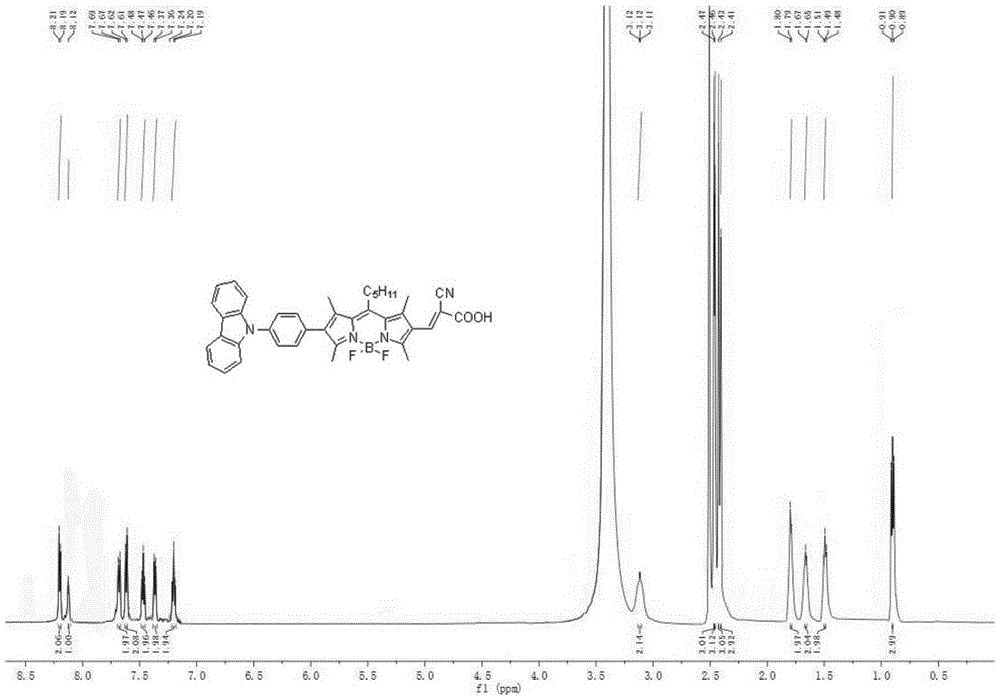
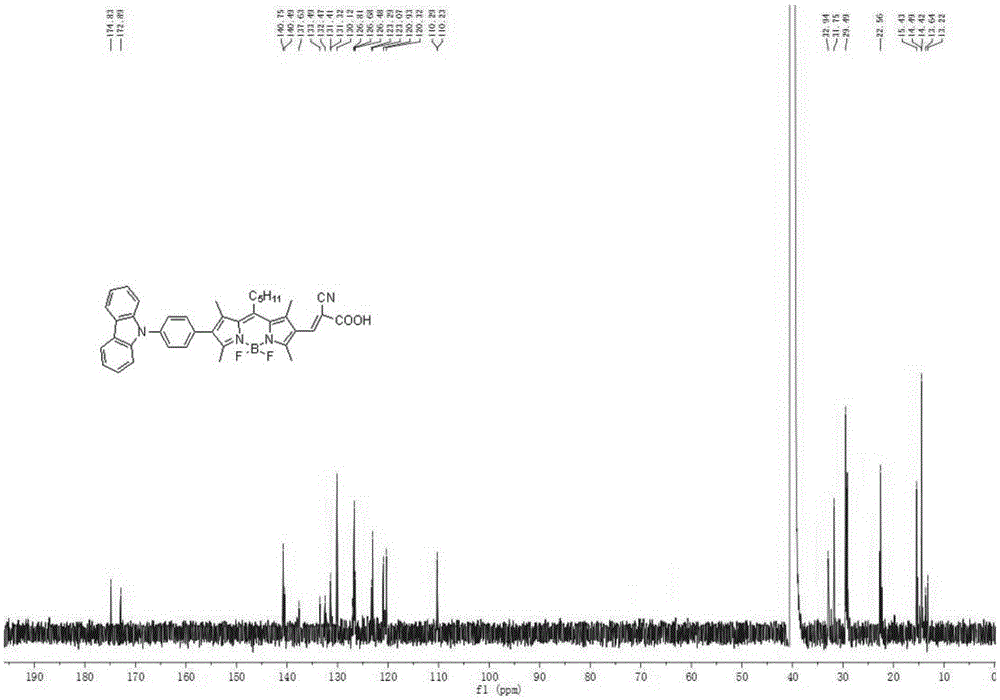
[0109] A method similar to synthetic dye 1 was used to obtain 150 mg of purple-red solid powder with a yield of 78%. 1 HNMR(600MHz,DMSO)δ: 8.21(d,J=7.7Hz,2H), 8.12(s,1H), 7.67-7.69(d,J=7.7Hz,2H), 7.62(d,J=7.7Hz, 2H), 7.47(d,J=9.8Hz,2H),7.36(d,J=7.2Hz,2H),7.19-7.24(t,J=7.3Hz,2H),3.12(s,2H),2.47( s, 3H), 2.46(s, 3H), 2.43(s, 3H), 2.41(s, 3H), 1.79(m, 2H), 1.65(m, 2H), 1.49(m, 2H), 0.90(t ,J=7.0Hz,3H). 13 CNMR (151MHz, DMSO) δ: 174.83, 172.89, 140.75, 140.49, 137.63, 133.49, 132.47, 131.41, 131.32, 130.12, 126.81, 126.68, 126.48, 123.29, 123.07, 120.93, 120.32, 110.29, 110.23, 32.94, 31.75, 29.49,22.56,15.43,14.49,14.42,13.64,13.22.MALDI-TOF-MS,m / z:calcdforC 42 H 49 BF 2 N 4 O 2 [M] + :690.400,found:690.251.
Embodiment 3
[0111] Synthesis of Dye3
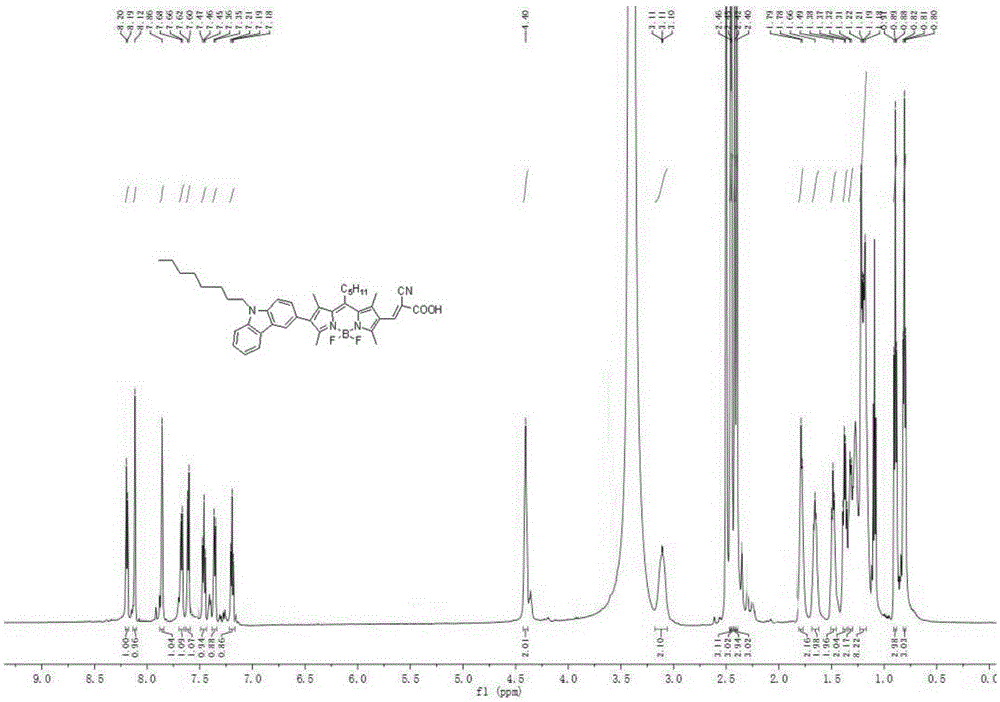
[0112] A method similar to synthetic dye 1 was used to obtain 145 mg of purple-red solid powder with a yield of 75%. 1 HNMR (600MHz, DMSO) δ: 8.20 (d, J = 6.5 Hz, 1H), 8.17 (d, J = 6.8 Hz, 1H), 7.91 (s, 1H), 7.60-7.55 (m, 2H), 7.46 ( t,J=7.3Hz,1H), 7.21(t,J=7.1Hz,1H), 7.09(d,J=6.7Hz,1H), 4.41(s,2H), 3.09(s,2H), 2.48( s,6H),2.41(s,6H),1.76(s,2H),1.65(s,2H),1.48(s,2H),1.37(d,J=6.6Hz,2H),1.23-1.13(m ,10H),0.90(t,J=6.8Hz,3H),0.78(t,J=6.6Hz,3H). 13 CNMR (151MHz, DMSO) δ: 155.41,151.10,148.38,140.81,140.66,139.06,137.58,135.96,132.25,131.06,130.20,126.32,126.03,122.34,121.75,121.27,120.83,120.72,119.31,111.52,109.77, 51.60,32.81,32.35,31.57,29.26,29.23,29.11,29.08,27.05,22.50,22.29,15.40,14.77,14.35,14.32,13.92,13.13.MALDI-TOF-MS, m / z:calcdforC 42 H 49 BF 2 N 4 O 2 [M] + :690.400,found:690.612.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com