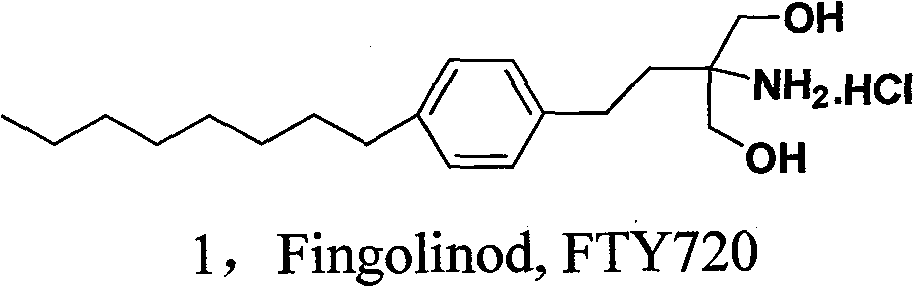
Novel synthesis method of fingolimod hydrochloride
A technology of fingolimod hydrochloride and a new method, applied in the new synthesis field of multiple sclerosis treatment drug fingolimod hydrochloride, can solve the problems of instability, difficult post-processing, long reaction route and the like, and achieve mild reaction conditions , Good use value, easy post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
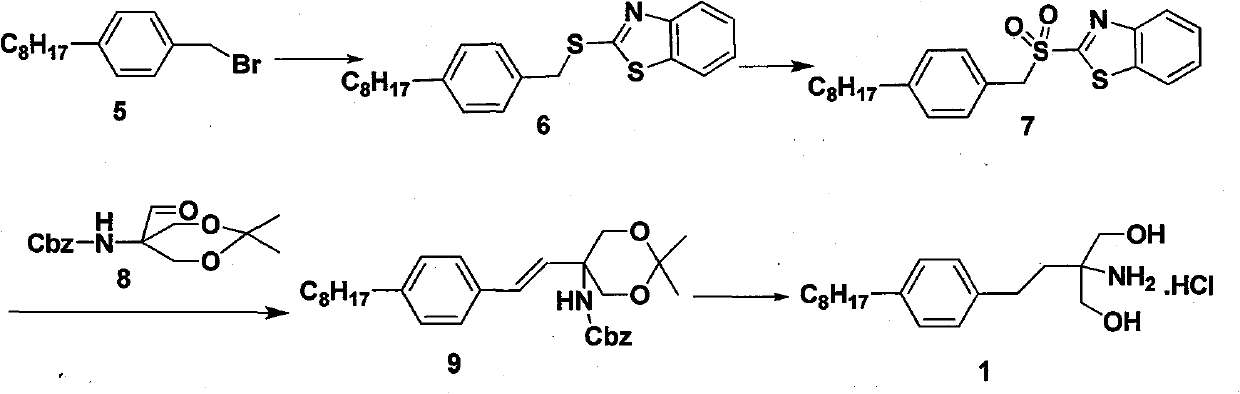
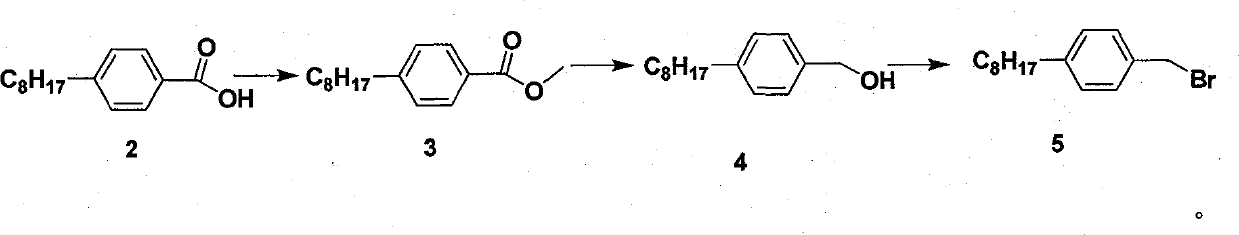
[0029] Dissolve p-4-n-octylbenzyl bromide (10.0g, 35.5mmol), 2-mercaptobenzothiazole (6.5g, 39mmol) and anhydrous potassium carbonate (5.4g, 39mmol) in 100ml of DMF, heat to reflux for 3 hours, When the reaction is complete, stop the reaction. After cooling, 100ml of water was added and extracted with ethyl acetate. The organic phases were combined, washed with water and saturated brine, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure to obtain 13.0 g of compound (6), a pale yellow solid, Mp: 52-54° C.; yield: 99%.
[0030] 1 H NMR (500HMz, CDCl3): 0.87(t, 3H), 1.26-1.29(m, 10H), 1.57-1.59(m, 2H), 2.57(t, 2H), 4.58(s, 2H), 7.14(d , J=8Hz, 2H), 7.25-7.31(m, 1H), 7.35(d, J=8Hz, 2H), 7.40-7.43(m, 1H), 7.74(d, J=8Hz, 1H), 7.89( d, J = 8 Hz, 1H).
[0031] Two, the synthesis of compound (7)
Embodiment 2
[0033] Compound (6) (7.4g, 20mmol) was dissolved in 150ml of chloroform, and m-chloroperoxybenzoic acid (14.8g, 60mmol) was added in 3 batches under ice-cooling conditions. After the addition, the ice bath was removed, and the mixture was stirred at room temperature for 4 hours, and the reaction was completed. Sequentially wash with saturated sodium bicarbonate, saturated sodium thiosulfate, water, saturated brine, and dry over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the obtained light yellow solid was recrystallized from ethyl acetate to obtain 6.8 g of the product, compound (7), as a light yellow solid, yield: 85%.
Embodiment 3
[0035] Compound (6) (7.4 g, 20 mmol) was dissolved in 30 ml of acetic acid, and 30% hydrogen peroxide (5.0 g, 42 mmol) was added dropwise under ice-cooling conditions. Then remove the ice bath, stir at room temperature for 20 hours, pour into 100ml of water after the reaction, extract with ethyl acetate (30ml*3), combine the organic layers, wash with saturated sodium bicarbonate, saturated sodium thiosulfate, water, and saturated salt Washed with water, dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the obtained light yellow solid was recrystallized from ethyl acetate to obtain the product, compound (7), 6.0 g, light yellow solid, yield: 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com