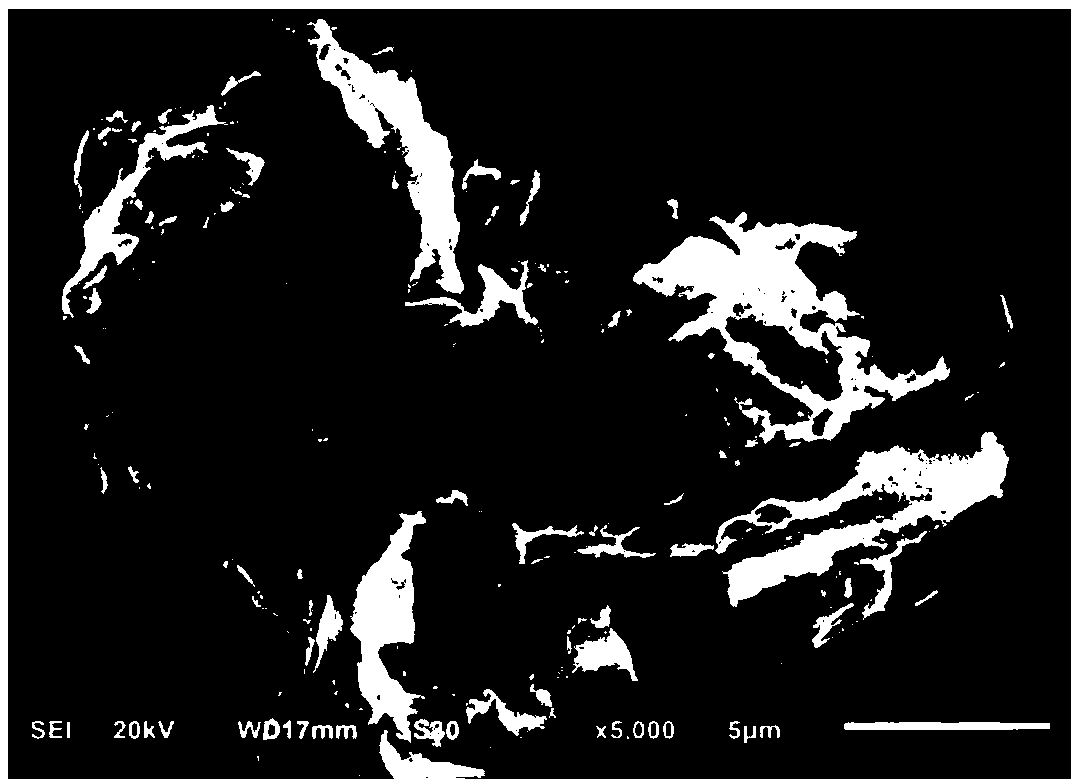
Preparation method of carbon nitride nanosheet catalyst and Knoevenagel condensation reaction method based on catalyst
A nanosheet and catalyst technology, applied in the field of catalyst preparation, can solve the problems of high reaction temperature, long reaction time and low conversion rate, and achieve the effects of mild reaction conditions, high activity and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] A kind of preparation method of carbon nitride nanosheet catalyst of the present invention, comprises the following steps:
[0027] 1) Mix the chloride salt, carbon and nitrogen source and solvent according to the mass ratio of (0.1~28):(2~8):(100~500) and stir to form a mixture; the chloride salt is LiCl, NaCl, KCl One, the carbon and nitrogen source is one of melamine, dicyandiamide or urea, and the solvent is deionized water;
[0028] 2) Rotate the mixture at a temperature of 60-80° C. to remove the solvent, and dry to form a mixture A;
[0029] 3) Put the mixture A in a muffle furnace to roast to form the mixture B, the roasting temperature is 500-600°C, the heating rate is 1-10°C / min, and the roasting time is 2-6h;
[0030] 4) The mixture B was washed with water several times, separated and dried to obtain a carbon nitride nanosheet catalyst.
[0031] In the following examples, the prepared carbon nitride nanosheet catalyst is represented by C 3 N 4 -NS-carbon ...
Embodiment 1
[0033] 4.0g melamine, 0.627g LiCl (14.8mmol) and 100g deionized water were mixed and stirred for 10min, and the solvent was removed by rotary evaporation at 60°C to form a mixture A; the mixture A was placed in a muffle furnace, and the temperature was raised at a rate of 2°C / min To 550°C, keep warm for 4h to form mixture B; soak mixture B in deionized water for 12h, suction filter, wash and dry to obtain C 3 N 4 -NS-melamine-LiCl-550-0.627 catalyst.
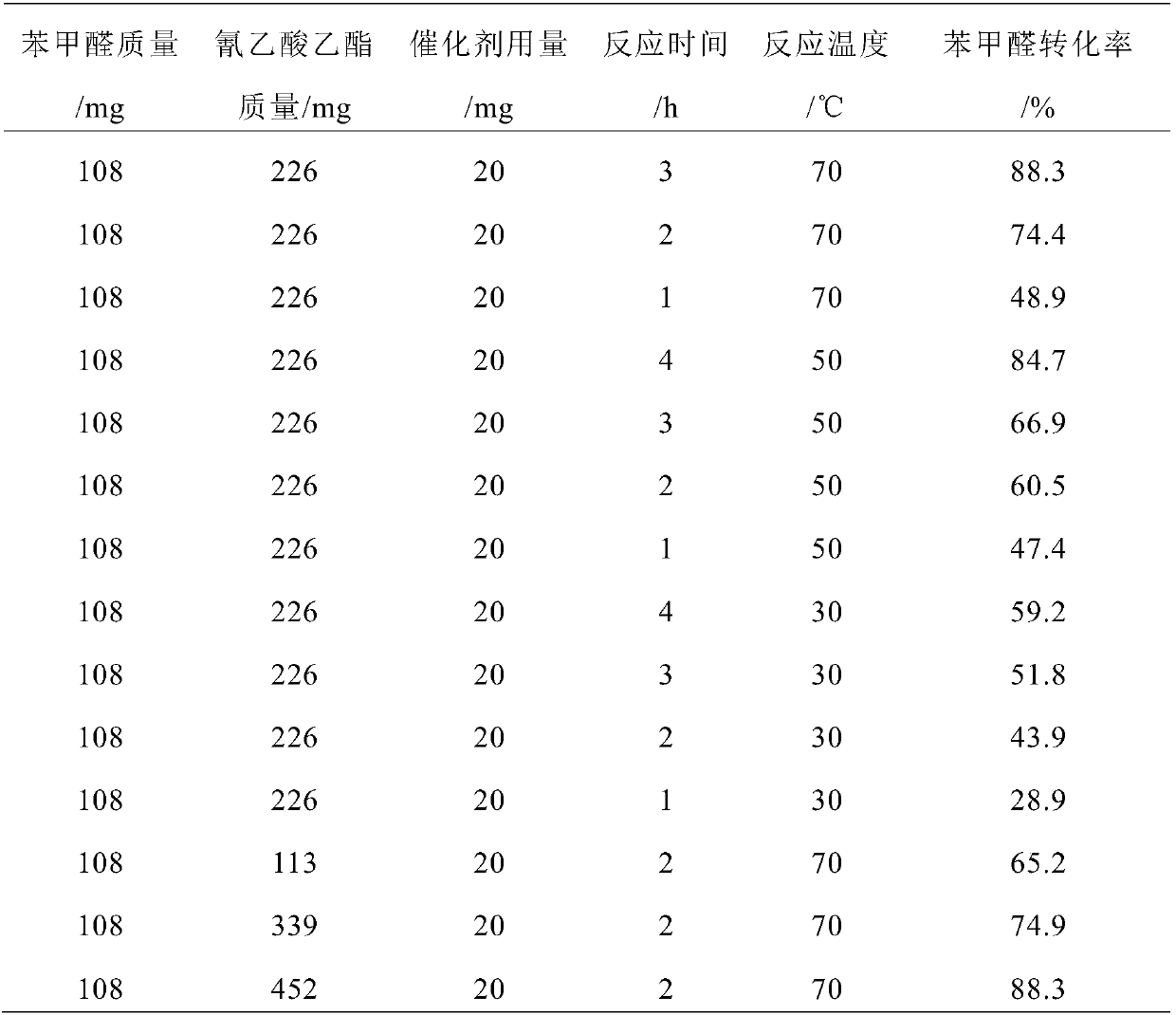
[0034] Take the above C 3 N 4 -NS-Melamine-LiCl-550-0.627 Catalyst 20mg for activity test, take reaction raw material benzaldehyde 108mg (1mmol), ethyl cyanoacetate 226mg (2mmol), n-butanol 4g in the flask, put the flask into the set temperature In a water bath at 70°C, start stirring, and react for 4 hours. After the set reaction time, stop the reaction. The reaction solution was centrifuged, and the supernatant was taken for gas chromatography analysis. The experimental results show that the conversion rate of benzaldehy...
Embodiment 2
[0036] 4.0g melamine, 0.864g NaCl (14.8mmol) and 100g deionized water were mixed and stirred for 10min, and the solvent was removed by rotary evaporation at 60°C to form a mixture A; the mixture A was placed in a muffle furnace, and the temperature was raised at a rate of 2°C / min To 550°C, keep warm for 4h to form mixture B; soak mixture B in deionized water for 12h, suction filter, wash and dry to obtain C 3 N 4 -NS-melamine-NaCl-550-0.864 catalyst.
[0037] With reference to the active testing method of catalyst in embodiment 1, get above-mentioned C 3 N 4-NS-melamine-NaCl-550-0.864 catalyst 20 mg was tested for activity, the experimental results showed that the conversion rate of benzaldehyde was 96.8%, and the selectivity of ethyl α-cyanocinnamate was 100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com