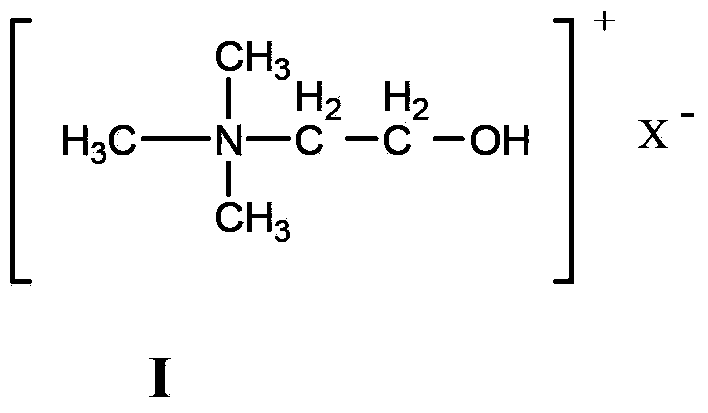
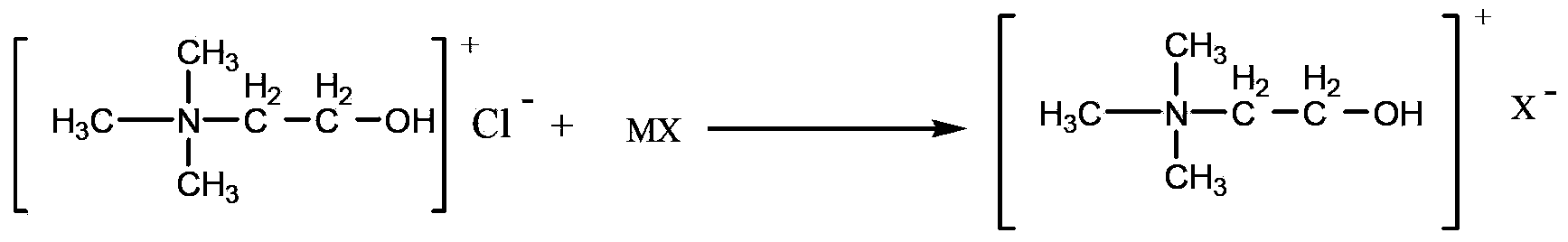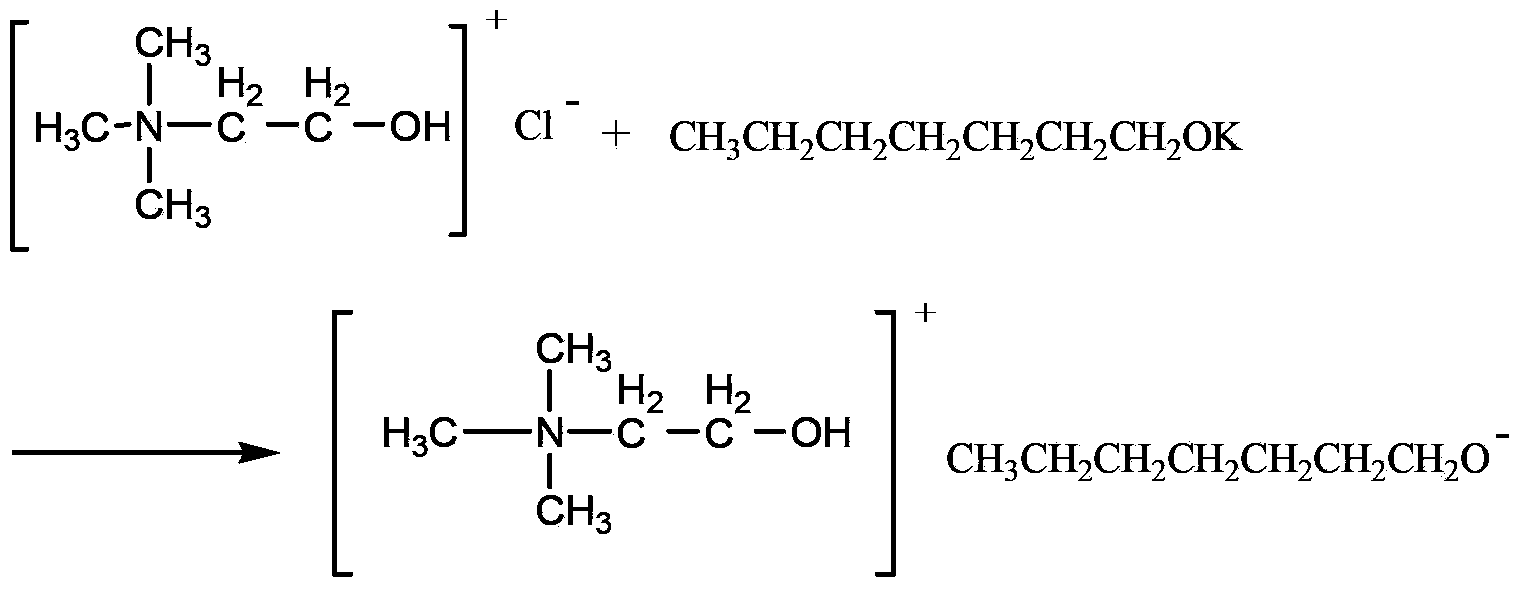Method for catalyzing Knoevenagel condensation reaction by using function ion liquid
A technology of functional ionic liquids and condensation reactions, applied in chemical instruments and methods, preparation of organic compounds, catalysts for physical/chemical processes, etc., can solve problems such as the use of toxic and harmful solvents, cumbersome post-reaction treatment, long reaction time, etc., to achieve Inexpensive, reusable, adjustable molecular structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add benzaldehyde (5mmol), malononitrile (6mmol), 4mL water, and 0.25mmol ionic liquid [Ch][OMe] into a 50mL single-necked flask in sequence, stir at room temperature for 1 minute, TLC detection, the raw materials disappeared, filtered, and vacuum-dried Cake obtains product, yield 98%, content 99%.
[0025] 2-(Phenylmethylene)-malononitrile:white solid;mp79-80℃; 1 H NMR (400 MHz, CDCl 3 )(ppm):7.91(d,2H,J=7.6Hz,ArH),7.79(s,1H,C=CH),7.64(t,1H,J=7.6Hz,ArH),7.27(t,2H, J=7.6Hz,ArH),7.27(t,2H,J=7.6Hz,ArH); 13 CNMR (100MHz, CDCl 3 )(ppm): 160.0, 134.6, 130.9, 130.7, 129.6, 113.7, 112.5, 82.7.
Embodiment 2
[0027] Add p-tolualdehyde (5mmol), malononitrile (6mmol), 4mL water, and 0.25mmol ionic liquid [Ch][OMe] into a 50mL single-necked bottle in turn, stir at room temperature for 1 minute, TLC detection, raw materials disappear, filter, Vacuum drying the filter cake to obtain the product with a yield of 99% and a content of 98%.
[0028] 2-(4-Methylphenylmethylene)malononitrile:white solid;mp134-135℃; 1 HNMR (400MHz, CDCl 3 )(ppm):7.82(d,2H,J=8Hz,ArH),7.73(s,1H,C=CH),7.34(d,2H,J=8Hz,ArH),2.46(s,3H,CH 3 ); 13 C NMR (100MHz, CDCl 3 )(ppm): 159.8, 146.4, 130.9, 130.3, 128.4, 114.0, 112.8, 81.1, 22.0.
Embodiment 3
[0030] Add p-nitrobenzaldehyde (5mmol), malononitrile (7.5mmol), 4mL water, and 5mmol ionic liquid [Ch][OMe] into a 50mL single-necked bottle in sequence, stir at room temperature for 0.5 minutes, TLC detection, raw materials disappear, filter , Vacuum drying the filter cake to obtain the product with a yield of 87% and a content of 97%.
[0031] 2-(4-Nitrophenylmethylene)malononitrile:yellow solid;mp136-138℃; 1 HNMR (400MHz, CDCl 3 )(ppm):8.46(s,1H,C=CH),8.32-8.29(d,1H,m,ArH),7.91-7.88(m,1H,ArH),7.84-7.80(m,2H,ArH) ; 13 C NMR (100MHz, CDCl 3 )(ppm): 158.9, 146.7, 134.9, 133.4, 130.4, 126.6, 125.8, 112.2, 110.9, 88.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com