Synthetic methods of chiral aryloxy propanol amine compounds and salts thereof
An aryloxypropanolamine and a synthesis method technology are applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., can solve the problems of high toxic and side effects, low efficacy, poor selectivity, etc. Simple, high product yield, good optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
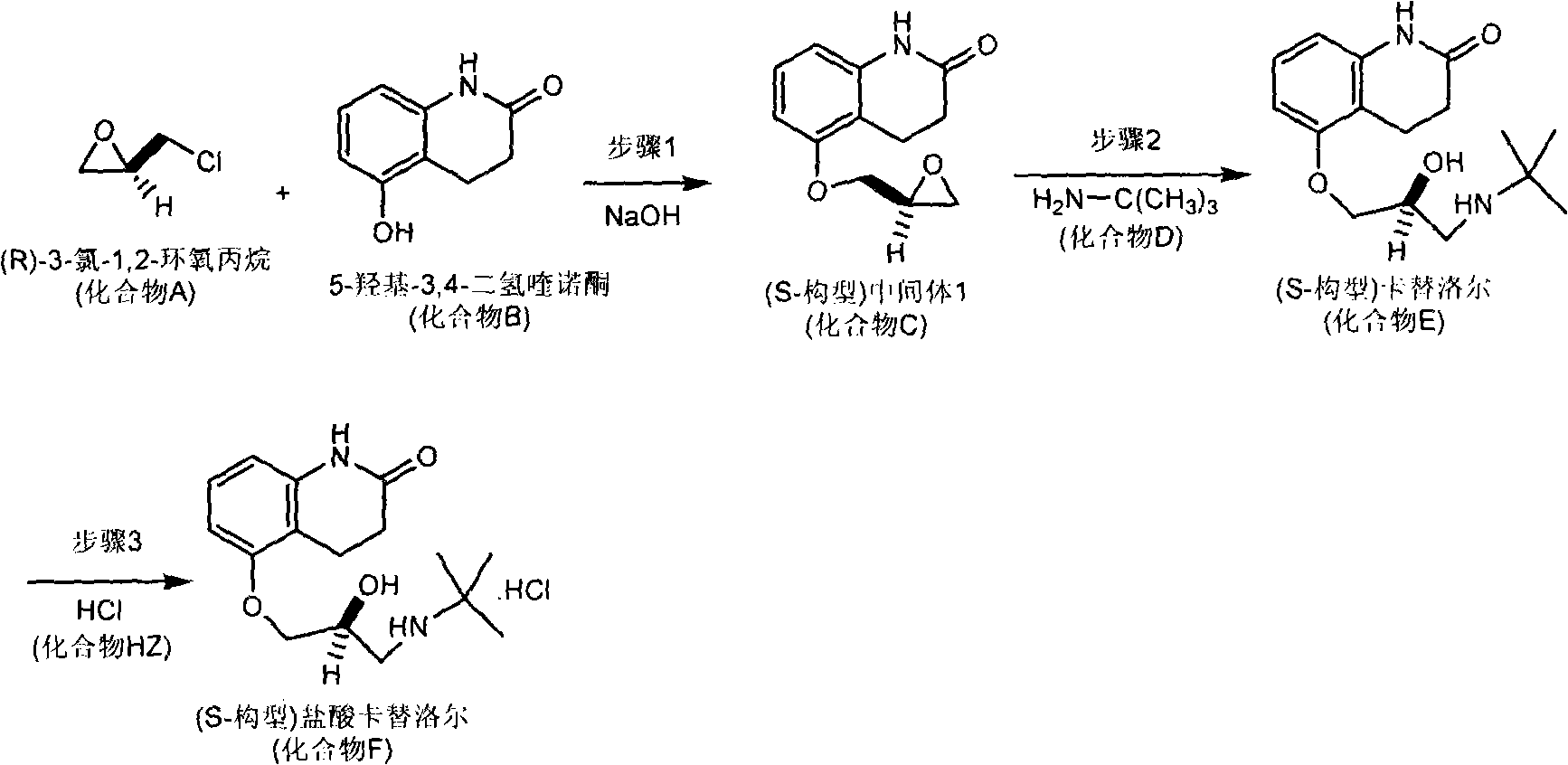
[0039] Embodiment 1: the preparation of S configuration carteolol hydrochloride
[0040] The synthetic route of S-configuration carteolol hydrochloride is attached figure 1 .
[0041] Step 1, etherification reaction: In the reactor, add 1.85g of (R) 3-chloro-1,2-epoxypropylene (compound A in the general formula), 1.63g of 5-hydroxyl-3,4- Dihydroquinolone (compound B in the general formula), 3.6 mL of water, 2.3 mL of N,N-dimethylformamide, stirred at room temperature for 5 minutes. An aqueous solution containing 0.8 g of sodium hydroxide was added, crystals were precipitated in the reaction solution, and the reaction was continued to stir at room temperature for 24 hours, and the reaction was stopped. The solid product was collected by filtration and recrystallized with acetone to obtain (S-configuration) intermediate 1 (compound C in the general formula), which was dried and weighed 1.75g, with a yield of 80% and an optical purity e.e. value of 99.5%.
[0042] Step 2, amin...
Embodiment 2
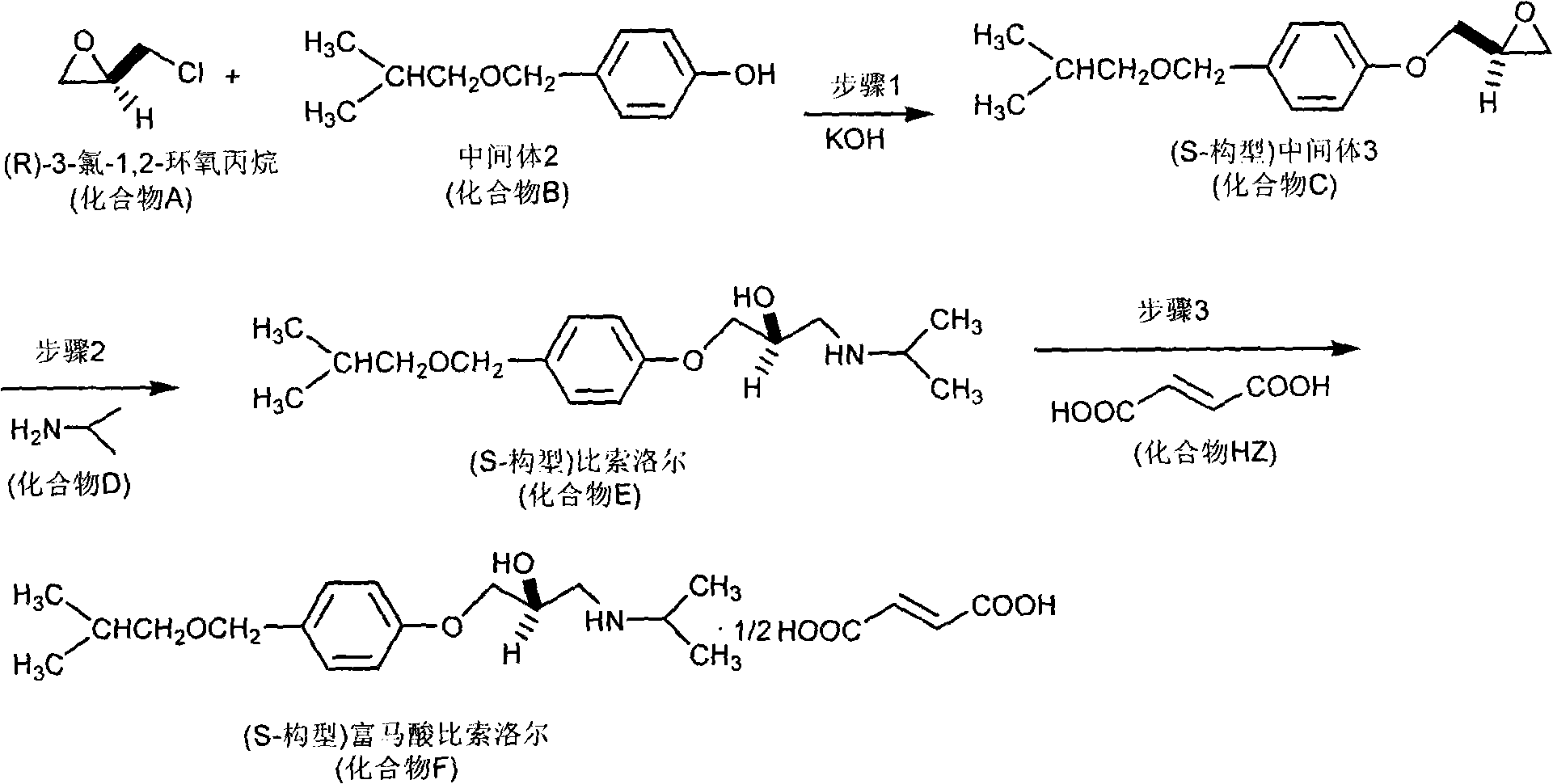
[0044] Embodiment 2: Preparation of S configuration bisoprolol fumarate
[0045] The synthetic route of S configuration bisoprolol fumarate is attached figure 2 .
[0046] Step 1, etherification reaction: In the reactor, add 1.80g of intermediate 2 (compound B in the general formula), 2.0mL of dimethyl sulfoxide, 2.00g of potassium hydroxide powder, and stir at room temperature for 5 minutes . Slowly add 1.85 g of (R) 3-chloro-1,2-propylene oxide (compound A in the general formula) dropwise, stir at room temperature for about 5 hours, and stop the reaction. 50 mL of water was added to the reaction liquid, and then extracted three times with 30 mL of dichloromethane, the organic phases were combined, and the solvent was recovered under reduced pressure to obtain a crude product. The crude product was recrystallized from acetone to obtain (S-configuration) intermediate 3 (compound C in the general formula), which was dried and weighed 1.98g with a yield of 84.0% and an optic...
Embodiment 3
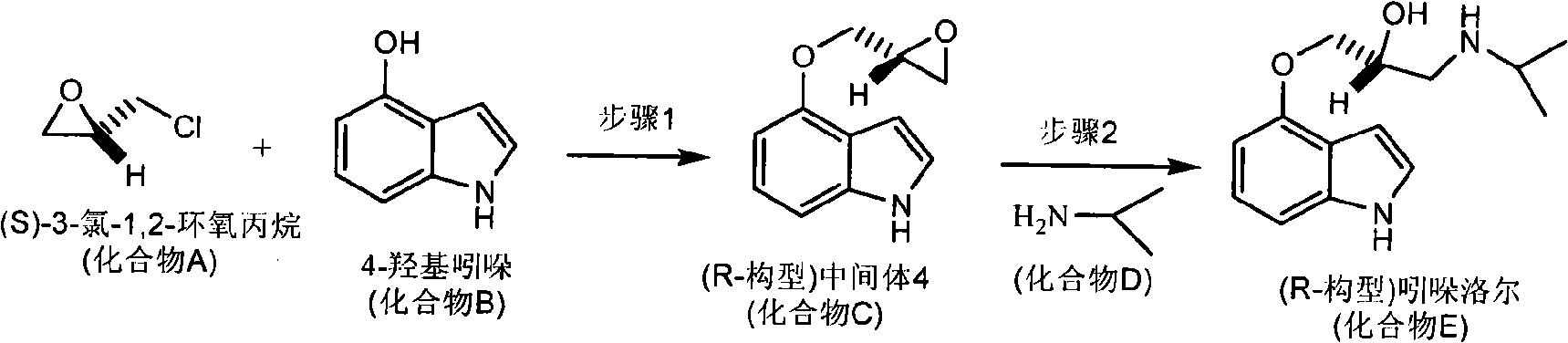
[0049] Embodiment 3: the preparation of R configuration pindolol
[0050] The synthetic route of R configuration pindolol is attached image 3 .
[0051] Step 1, etherification reaction: In the reactor, 13.3g 4-oxindole (compound B in the general formula) was added to 15.5mL (S)-3-chloro-1,2-epoxypropane (general formula In the mixed solution of compound A) and 50.0 mL of ethanol, slowly add an aqueous solution containing 2.70 g of potassium carbonate dropwise at room temperature, and react for 6 hours under temperature control at 30-40° C. after the dropping is completed. Add 50.0 mL of water, adjust the pH to neutral with hydrochloric acid, recover ethanol to precipitate solids, filter, wash with water, and dry to obtain 15.56 g (R-configuration) of intermediate 4 (compound C in the general formula), with a yield of 82.3%. The purity e.e. value is 98.5%.
[0052] Step 2, amination reaction: In a reactor, add 10.00 g (R-configuration) of intermediate 4 (compound C in the g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com