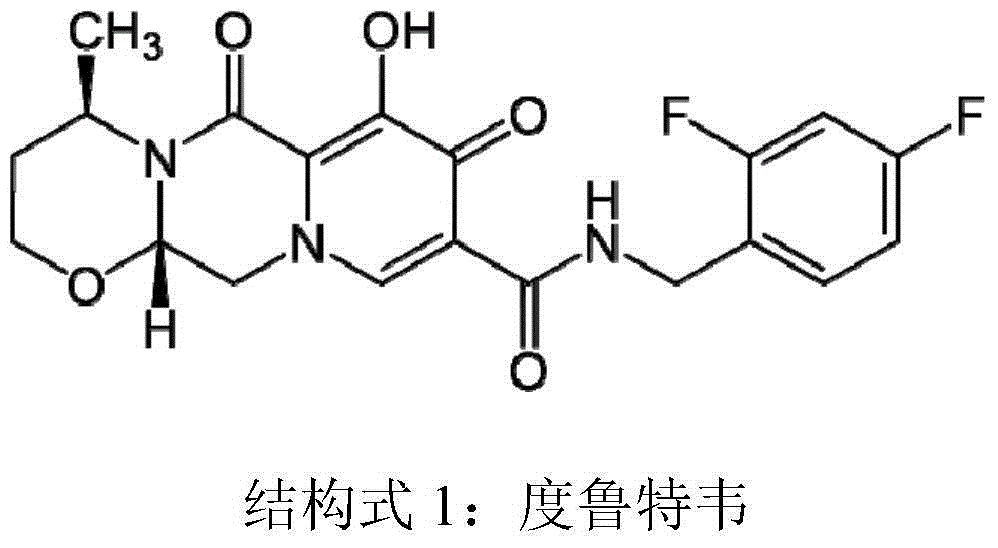
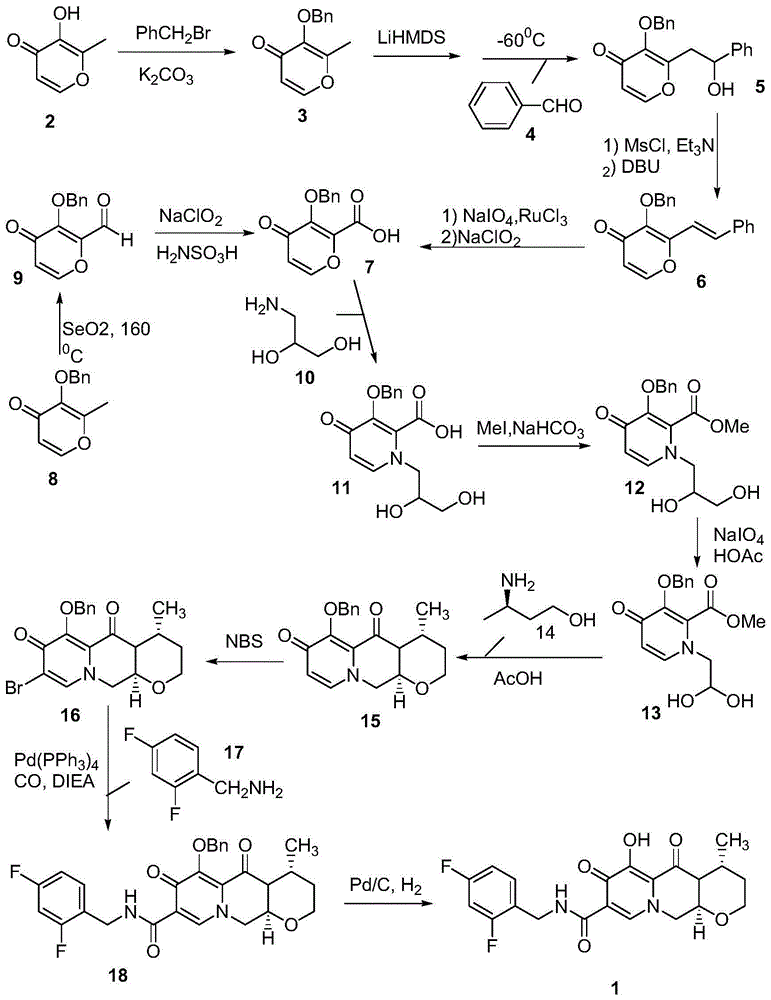
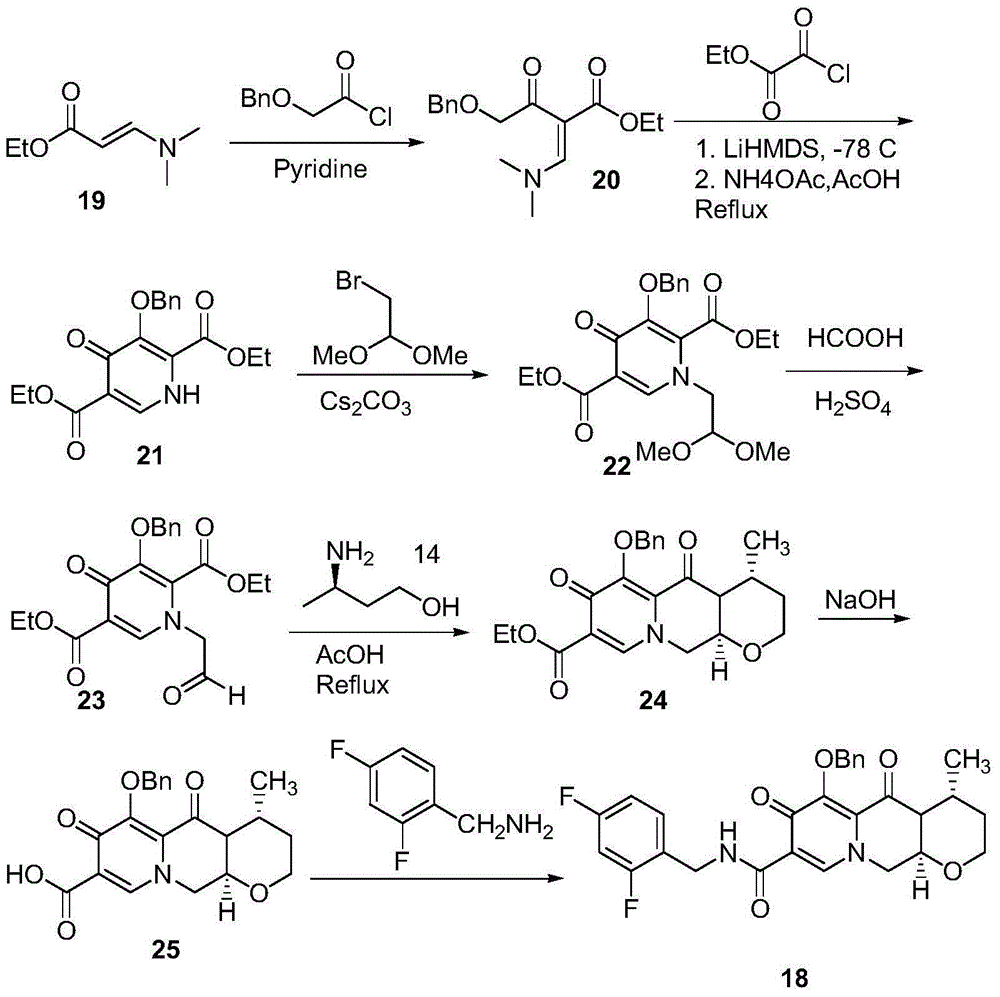
Preparation method for optical activity active 3-amino butanol and optical activity 3-amino butyric acid
An alkyl and chiral carbon technology, applied in the preparation of aminohydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of low yield, low purity, high toxicity of raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1: the synthesis of ethyl 3-acetamidocrotonate (p-toluenesulfonic acid catalysis)
[0105] Add 52 kilograms of ethyl acetoacetate, 35 kilograms of acetamide and 300L cyclohexane in a 500-liter reactor, and stir thoroughly. Then add 3 kilograms of p-toluenesulfonic acid, and slowly heat up to reflux and divide water through the water separator. When the reaction was about 24 hours, the sample was detected by GC, and when the ratio of product and raw material reached 9:1, the stirring was stopped and left to stand. Separate the layers while hot, cool the upper layer to room temperature, then add 50 kg of water to wash and separate the layers, concentrate the upper organic layer until a large amount of solid precipitates, filter to obtain white crystals, and vacuum dry to obtain 37.6 kg of white solids, with a yield of 55%. Melting point: 64-65°C. Gas phase detection purity: 98.7%.
[0106] 1 H NMR (300MHz, CDCl3): δ1.28(t, J=7.2Hz, 3H), 2.14(s, 3H), 2.38(s,...
Embodiment 2
[0107] Embodiment 2: Synthesis of ethyl 3-acetamidocrotonate (catalyzed by boron trifluoride ether)
[0108] Add 500 grams of ethyl acetoacetate, 340 grams of acetamide and 3 liters of cyclohexane into a 5-liter round bottom flask, and stir well. Then add 25 grams of boron trifluoride diethyl ether, and slowly heat up to reflux and separate water through a water separator. When the reaction was about 24 hours, the sample was detected by GC, and when the ratio of product and raw material reached 9:1, the stirring was stopped and left to stand. The layers were separated while hot, the upper layer was cooled to room temperature, and then 500 ml of water was added to wash the layers. The upper organic layer was concentrated until a large amount of solids were precipitated. The white crystals were obtained by filtration and vacuum-dried to obtain 394 g of white solids, with a yield of 61%. Melting point: 64.5-65°C. Gas phase detection purity: 99.1%.
[0109] 1 H NMR (300MHz, CD...
Embodiment 3
[0110] Embodiment 3: the synthesis of (R)-3-acetylaminobutyric acid ethyl ester
[0111] Add 200 grams of ethyl 3-acetylaminocrotonate and 800 milliliters of methanol into a 2L hydrogenation reactor, and replace with nitrogen for 30 minutes; then 83 milligrams of Rh(SSRR-TangPhos)(COD)BF 4 Add to the reaction kettle. Carefully add hydrogen, and maintain 1-5 atmospheric pressure at room temperature for 20-24 hours; the reaction of the raw materials was detected by gas chromatography, and the discharge was concentrated to obtain 186 grams of oil, with a yield of 92%, ee99.4%. ee Gas phase detection conditions: Astec CHIRALDEXTM B-DM 30mx0.32mmx0.12um chromatographic column. Carrier gas: nitrogen; flow rate: 1.5mL / min; column temperature: 110°C constant temperature; retention time: R‐3‐acetylaminobutyrate 14.7 minutes, S-3‐acetylaminobutyrate 12.8 minutes.
[0112] 1 H NMR (300MHz, CDCl 3 ):δ6.15(brs,1H),4.39-4.31(m,1H),4.21(q,7.2Hz,2H),2.52(dd,2.8,5.3Hz,2H),1.96(s,3H),1.27 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com