Preparation method for free-state edoxaban
A technology of edoxaban and free state, which is applied in the field of compound synthesis, can solve the problems of unfavorable process preparation in purification methods, solidification of reaction liquid, and easy inclusion of impurities, etc., and achieve the effect of improved crystallization method, mild reaction conditions, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
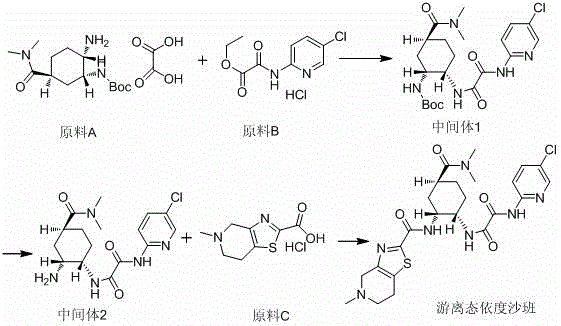
[0021] A preparation method of free edoxaban, the synthetic route is as follows:
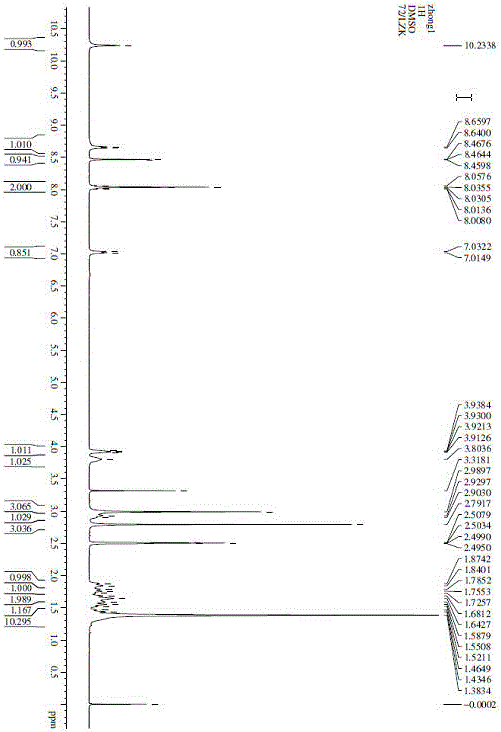
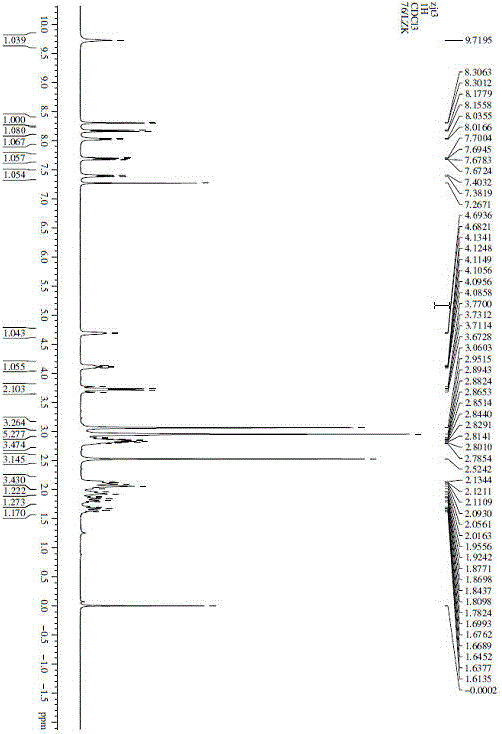
[0022]
[0023] 1. Preparation of Intermediate 1:
[0024]
Embodiment 1
[0025] Example 1: Suspend raw material B (25.5g, 0.096mol) in acetonitrile (300mL), add the first amount of triethylamine (9.6g, 0.096mol) dropwise at 10°C±2°C, and then add raw material A (30g, 0.0810mol), add dropwise the second amount of triethylamine (39g, 0.384mol), raise the temperature to 60°C±5°C, stir at 60°C±5°C for 6-8 hours, then cool down to room temperature, add the first A certain amount of water (300mL) was used to quench the reaction; after stirring for 1-1.5 hours at 10°C±2°C, a second amount of water (600ml) was added, filtered, and the resulting solid was washed and purified to obtain the compound intermediate 1 (34.8 g, yield 93%, product purity > 95%);
Embodiment 2
[0026] Example 2: Suspend raw material B (25.5g, 0.096mol) in acetonitrile (300mL), add the first amount of triethylamine (9.6g, 0.096mol) dropwise at 10°C±2°C, and then add raw material A (30g, 0.0810mol), drop the second amount of triethylamine (39g, 0.384mol), raise the temperature to 60℃±5℃, and stir at this temperature for 6-8 hours, then cool down to room temperature, add water (900mL ) to quench the reaction; after stirring for 1-1.5 hours at 10°C ± 2°C, filter, and the obtained solid was washed and purified, and the spot plate showed that raw material B was included, and recrystallization and purification made the preparation process cumbersome, and intermediate 1 (30g , Yield 80%), the yield decreases.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com